- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Autoradiographic 3H-Gaboxadol Receptor Binding Protocol
Published: Vol 3, Iss 23, Dec 5, 2013 DOI: 10.21769/BioProtoc.989 Views: 7889
Reviewed by: Xuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enrichment of Membrane Proteins for Downstream Analysis Using Styrene Maleic Acid Lipid Particles (SMALPs) Extraction
Benedict Dirnberger [...] Kathryn S. Lilley
Aug 5, 2023 2978 Views

Establishment of Human PD-1/PD-L1 Blockade Assay Based on Surface Plasmon Resonance (SPR) Biosensor
Tess Puopolo [...] Chang Liu
Aug 5, 2023 2842 Views

A Computational Workflow for Membrane Protein–Ligand Interaction Studies: Focus on α5-Containing GABA (A) Receptors
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
Nov 20, 2025 2123 Views
Abstract
Gaboxadol (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol, THIP), a GABAA receptor δ-subunit specific agonist, when present at low (μM) concentrations, preferentially binds and activates extrasynaptic (non-γ2, δ-subunit-containing) GABAARs (Storustovu and Ebert, 2006; Richardson et al., 2011, 2013).
In this prototype saturation binding experiment, a series of concentrations of [3H]gaboxadol (5, 10, 25, 50, 75, 100, 250 and 400 nM) will be used. GABA at 200 μM will be added into binding mixtures as a cold displacer for [3H]gaboxadol. Slide mailers are used and each requires 7 ml binding mixture. Pre-, post-washing and binding buffer is 50 mM Tris-Citrate (pH 7.1). The detailed procedure is outlined below.
Materials and Reagents
- Ice
- [3H]gaboxadol (Gift from Merck & Co.)
- GABA (Sigma-Aldrich, catalog number: A2129 )
- Incubation buffer: 50 mM Tris-Citrate buffer (pH 7.1)
- 0.1 M PBS (Phosphate buffer saline, pH 7.4) Binding mixture (see Recipes)
Equipment
- Slide mailer (Fisher Scientific, model: HS15986 )
- Labeled foil
- Cryostat (Leica Microsystems, model: CM1850 )
- -20 ℃ freezer
- Scintillation counter
- Ruler
- Diamond scribe
- Double-sided tape
- Phosphor transcreens
- Cyclone Phosphor Imager (PerkinElmer)
Software
- OptiQuant Version 4.0 (PerkinElmer)
- GraphPad Radioactive Calculator (GraphPad Software)
Procedure
- Animal brains will be removed after a quick decapitation and dipped in the PBS slush (0.1 M, pH 7.4) to remove surface blood. Once the brain tissue is frozen in dry ice, it can be wrapped in labeled foil and stored at -80 °C.
- Sixteen micron thickness frozen sections will be cut in a cryostat, collected on slides and stored at -20 °C freezer for less than 48 h.
- Slides will be brought to room temperature on the experiment day; DO NOT open the box until slides have equilibrated to room temperature (about 20 min at room temperature) in order to avoid excessive moisture on tissue sections. Afterwards, slides will be selected to be used for the experiment.
- Wash with 50 mM Tris-Citrate buffer (pH 7.1) at 4 °C for 5 min three times.
- Let slides dry at room temperature (leave slides for 45 min to 1 h or longer).
- While waiting for the slides to dry
- Make “Shoot for” solutions:
- Theoretical “Shoot for”: “Shoot for” is the stock solution used to make a series of dilutions for receptor saturation analysis. The range of [3H]gaboxadol concentrations will be determined based on the literature and previous experiments. The highest concentration will then be used to calculate a theoretical “Shoot for”. For example, if the highest concentration used in the experiment is 400 nM of [3H]gaboxadol, the total volume added to the slide mailer is 7 ml. The volume of the 10X stock displacer solution to be added to the mailer is 0.7 ml; therefore, 6.3 ml of X concentration of the “Shoot for” will be needed. Thus, the “Shoot for” will be calculated as:
X = (400 nM*7 ml)/6.3 ml, and X = 444.44 nM.
- Volume (V) of the original [3H]gaboxadol required to make the theoretical “Shoot for”:
V (μl) = Vt * X * SA
SA is the radioactive ligand’s specific activity (Ci/mmol) provided by the manufacturer. In addition, the specific activity for the same ligand may vary between production batches;
X is the theoretical concentration of the “Shoot for” (nM);
Vt is the total volume of “Shoot for” needed to make the series dilution (L). Therefore, Vt = V1+V2+……+VHighest
For each concentration, there will be a “total” mailer (measuring total binding, displacer omitted) and a “blank” mailer (measuring non-specific binding, displacer added). For example, a series of concentrations of 5, 10, 25, 50, 75, 100, 250 and 400 nM [3H]gaboxadol are used and the total volume of the “Shoot for”:
Vt (L) = [(5 nM*7 ml/X)+(10 nM*7 ml/X)… +(400 nM*7 ml/X)]*2
1000
- The “Shoot for” solution will be made by adding V (μl) of the original [3H]gaboxadol to Vt-V amount of the incubation buffer.
- Theoretical “Shoot for”: “Shoot for” is the stock solution used to make a series of dilutions for receptor saturation analysis. The range of [3H]gaboxadol concentrations will be determined based on the literature and previous experiments. The highest concentration will then be used to calculate a theoretical “Shoot for”. For example, if the highest concentration used in the experiment is 400 nM of [3H]gaboxadol, the total volume added to the slide mailer is 7 ml. The volume of the 10X stock displacer solution to be added to the mailer is 0.7 ml; therefore, 6.3 ml of X concentration of the “Shoot for” will be needed. Thus, the “Shoot for” will be calculated as:
- Count “Shoot for” using Scintillation counter by aliquoting 10 μl of “shoot for” into 5 ml of scintillation fluid. Based on the counts, calculate the actual concentration of the “Shoot for” using GraphPad Radioactive Calculator. If the measured value is close to the theoretical concentration, continue to the next step. From this point forward, the actual concentration of the stock solution will be used for the dilution calculations.
- Pipetting required amount of 50 mM Tris-Citrate buffer to tubes labeled with Total or Blank of 5, 10, 25, 50, 75, 100, 250 and 400 nM of [3H]gaboxadol, add appropriate volume of the stock solution to make to the designated concentrations.
- Add 0.7 ml of 200 μM GABA into tubes labeled Blank.
- Count the counts of the mixture in 16 tubes. The actual concentration of [3H]gaboxadol in each tube will be calculated based on these counts.
- Make “Shoot for” solutions:
- Transfer mixtures from 16 tubes into the correctly labeled slide mailer.
- Drop slides into their designated mailers. Shake well, before leaving mailers in the refrigerator for 1 h at 4 °C.
- After incubation, slides will be washed twice in cold Tris-Citrate buffer for 10+10 sec, followed by cold dH2O for 10 sec.
- Let slides dry for 2-4 h or overnight.
- Break the slides: re-label the slides next to the sections, line up sections with a ruler and cut the slides using a diamond scribe. The slide end (with one section on it) will be taped using double-sided tape after being broken.
- Put the slides onto the phosphor transcreens; transcreen exposure time is about 48 h.
- Binding images are obtained by scanning the exposed phosphor transcreens using a Cyclone Phosphor Imager.
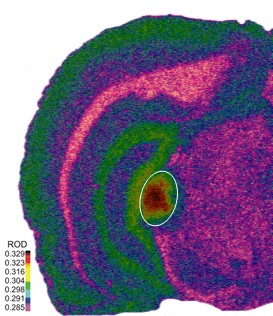
Figure 1. An autoradiographic image of [3H]gaboxadol on GABAA receptor binding in a four month old Fischer Brown Norway rat brain tissue (16 μm thickness). Highest binding area (circled) is the medial geniculate body, while hippocampus and cortex show moderate binding.
Recipes
- Binding mixture
Binding mixture consists of 50 mM Tris-Citrate buffer and [3H]gaboxadol with/without 200 μM GABA
Acknowledgments
This protocol is adapted from Richardson et al. (2011) and Richardson et al. (2013). Work supported by NIH DC000151 to DMC.
References
- Richardson, B. D., Ling, L. L., Uteshev, V. V. and Caspary, D. M. (2013). Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 33(3): 1218-1227a.
- Richardson, B. D., Ling, L. L., Uteshev, V. V. and Caspary, D. M. (2011). Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS One 6(1): e16508.
- Storustovu, S. I. and Ebert, B. (2006). Pharmacological characterization of agonists at delta-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther 316(3): 1351-1359.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ling, L. and Caspary, D. (2013). Autoradiographic 3H-Gaboxadol Receptor Binding Protocol. Bio-protocol 3(23): e989. DOI: 10.21769/BioProtoc.989.
-
Richardson, B. D., Ling, L. L., Uteshev, V. V. and Caspary, D. M. (2013). Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 33(3): 1218-1227a.
Category
Neuroscience > Cellular mechanisms > Receptor-ligand binding
Biochemistry > Protein > Interaction > Protein-ligand interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link