- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Implantation of Dkk-1-soaked Beads into the Neural Tube of Chicken Embryos
Published: Vol 3, Iss 21, Nov 5, 2013 DOI: 10.21769/BioProtoc.963 Views: 13006
Reviewed by: Xuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Open-book Preparations from Chick Embryos and DiI Labeling of Commissural Axons
Nicole H. Wilson and Esther T. Stoeckli
Jul 5, 2014 13186 Views

The Chick Chorioallantoic Membrane (CAM) Assay as a Three-dimensional Model to Study Autophagy in Cancer Cells
Félice A. Janser [...] Mario P. Tschan
Jul 5, 2019 10805 Views

Qualitative Detection of Lipid Peroxidation in Mosquito Larvae Using Schiff’s Reaction: A Simple Histochemical Tool for In Situ Assessment of Oxidative Damage
Antonella Cuniolo [...] María Victoria Martin
Feb 5, 2026 83 Views
Abstract
Chick embryos are known to be a powerful system to test gene function due to the “in vivo” accessibility, short time for results retrieval and the possibility to perform a large number of experiments. Synthetic micro-beads soaked in morphogenetic signals or receptor inhibitors can be implanted in selective embryo regions at precise developmental stages activating or blocking different signaling pathways. Here, we describe the manipulation of Wnt signaling pathway using Dkk-1-soaked micro-beads, implanted in ovo in the anterior part of the developing neural tube of chicken embryos; specifically at the prospective zona limitans intrathalamica at stage HH10.
Keywords: Experimental embryologyMaterials and Reagents
- Fertilized chick (Gallus gallus)
- Recombinant mouse Dkk-1 (R&D Systems, catalog number: 1765-DK )
- Heparin Acrylic beads (Sigma-Aldrich, catalog number: H5263 )
- Bovine Serum Albumine (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Pelikan Drawing Ink Black
- Silikon Peroxid (IDEX Health & Science, catalog number: SC0083ST )
- Tungsten wire (0.380 mm, 10 FT) (World Precision Instruments, catalog number: TGW1510 )
- Grid with concentric circles (NE42-21 mm) (Graticules LTD, Tonbridge Kent)
- Penicillin-Streptomycin (10,000 U/ml) (Gibco, catalog number: 15140-122 )
- NaCl (Sigma-Aldrich, catalog number: S-3014 )
- KCl (Sigma-Aldrich, catalog number: P-9541 )
- CaCl2.2H2O (Prolabo, catalog number: 22317297 )
- NaH2PO4.2H2O (Scharlab, catalog number: SO0334 )
- MgCl2.6H2O (Prolabo, catalog number: 27778293 )
- D(+) glucose anhydre (Prolabo, catalog number: 24370294 )
- 10x Phosphate-buffer saline (PBS) (see Recipes)
- 4% Paraformaldehyde (PFA) from 20% PFA (see Recipes)
- PBS/0.1% BSA (see Recipes)
- Tyrodes buffer (see Recipes)
- 0.5% Bicarbonate (see Recipes)
- 0.5% Glucose (see Recipes)
- Tyrodes supplemented (see Recipes)
Equipment
- 37 °C, 5% CO2 forced-air incubator (Novital, model: Covatutto 120-4V )
- Dissecting microscope (Leica Microsystems, model: MS5 )
- Butane burner (Butsir)
- Glass micropipet (homemade)
- Pasteur pipets (Deltalab, catalog number: 200001 )
- Silicon tube (IDEX Health & Science, catalog number: SC0083ST )
Procedure
- Fertilized chick (Gallus gallus) was incubated at 37 °C in a forced-air incubator. Chick embryos were developed until stage HH10 according to Hamburger and Hamilton (1951).
- Glass micropipets were prepared using a Bunsen burner. One side of the micropipet was introduced into a silicon tube that was used to aspirate solution while picking up each bead (Figure 1A-D). Tungsten wire was joined to a Pasteur pipet to handle easily (Figure 1E).
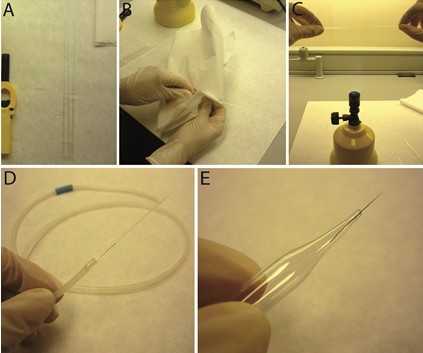
Figure 1. Micropipets preparation. C-D. Tight part of Pasteur pipets was used to prepare micropipets. E. Wide part was used as a handle for the tungsten wire. - Use forceps to selective heparin acrylic beads based on their size. A grid inserted in one ocular of the operating microscope was used to select beads of ~40 μm. Afterwards, beads were rinsed in PBS and then soaked in a solution of 25 μg/ml of Dkk-1 protein in PBS/0.1% BSA or in PBS overnight (o/n) at 4 °C.
- An opening in the shell was made with scissors. To counterstained the embryos in ovo, inject Indian ink (diluted 1:1 in Tyrode supplemented) under the blastoderm by a glass micropipet made with a Bunsen burner. The vitelline membrane that covers the embryo is slipped out with tungsten wire on the spot where microsurgery was to be performed (see Video 1).
Video 1. Counterstaining of chicken embryos in ovo - A grid with concentric circles was inserted in one ocular of the operating microscope. The working magnification used during microsurgery was 40x. This grid was positioned in relation to the embryo: the vertical axis followed the embryo ventral midline; the transversal axis was positioned by crossing the optic-diencephalic angle at both sides (Figure 2A) (Garcia-Lopez et al., 2004).
- Afterwards, the soaked-beads were cut to obtain half-beads using forceps. Then they were rinsed in PBS several times and one soaked half-bead implanted in the right side of the neural tube of embryos (Figure 2B). For control experiments, beads were soaked in PBS in the same manner.
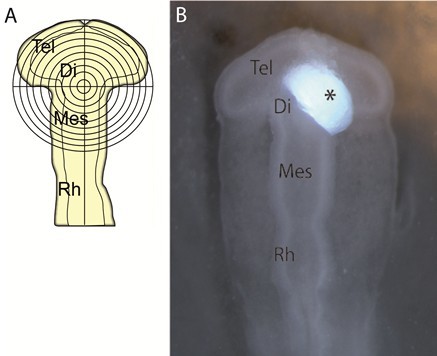
Figure 2. Bead-implanting procedure. A. Schematic representation of the grid positioned in relation to the embryo. B. Dkk-1 (black asterisk) or PBS-soaked beads were implanted into the prospective zona limitans intrathalamica of chick embryos at HH10. Abbreviations: Di, diencephalon; Mes, mesencephalon; Rh, rhombencephalon; Tel, telencephalon. - When the operation was completed, the opening in the eggshell was sealed with a piece of tape and incubated in horizontal position until the stage selected for fixation.
Recipes
- 10x PBS– phosphate-buffer saline
Mix 80 g of NaCl with 2 g of KCl, 14.4 g of Na2HPO4, 2.4 g of KH2PO4
Adjust pH to 7.4 with NaOH
Add dH2O to 1,000 ml
Filter sterilize (0.2 μm)
Stored at RT - 20% PFA- paraformaldehyde
Mix 600 ml of preheated 1x PBS at 65 °C with 200 g of PFA
Add PBS to 1,000 ml
Adjust pH to 7.4 with NaOH
Filter sterilize (0.2 μm)
Stored at -20 °C - PBS/0.1% BSA
Mix 0.1 g of BSA with 100 ml of 1x PBS - Tyrodes buffer
4 g NaCl
0.1 g KCl
0.13 g CaCl2.2H2O
0.028 g NaH2PO4.2H2O
0.022 g MgCl2.6H2O
Add dH2O to 300 ml, sterilize and stored at 4 °C - 0.5% Bicarbonate
0.5 g sodium hydrogen carbonate NaHCO3
Add dH2O to 100 ml, sterilize and stored at 4 °C - 0.5% Glucose
0.5 g D(+) glucose anhydre
Add dH2O to 100 ml, sterilize and stored at 4 °C - Tyrodes supplemented
30 ml Tyrodes buffer
10 ml 0.5% Glucose
10 ml 0.5% Bicarbonate
500 μl 10,000 U/ml penicillin-streptomycin
Acknowledgments
This protocol was adapted from the previously published studies, Crossley et al. (1996) and Vieira and Martinez, (2005), and it was performed by Martinez-Ferre et al. (2013). This work was supported by EUCOMMTOOLS Contract 261492, Spanish Ministry of Science and Innovation Grant BFU-2008-00588, Spanish Ministry of Education and Science-Universitary Professor Formation Grant AP2009-3644, Consolider Grant CSD2007-00023, Institute of Health Carlos III, Spanish Cell Therapy Network and Research Center of Mental Health, General Council of Valencia (Prometeo 2009/028 and 11/2011/042), and the Alicia Koplowith Fondation. We thank M. Ródenas for technical assistance.
References
- Crossley, P. H., Martinez, S. and Martin, G. R. (1996). Midbrain development induced by FGF8 in the chick embryo. Nature 380(6569): 66-68.
- Garcia-Lopez, R., Vieira, C., Echevarria, D. and Martinez, S. (2004). Fate map of the diencephalon and the zona limitans at the 10-somites stage in chick embryos. Dev Biol 268(2): 514-530.
- Hamburger, V. and Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. J Morphol 88(1): 49-92.
- Martinez-Ferre, A., Navarro-Garberi, M., Bueno, C. and Martinez, S. (2013). Wnt signal specifies the intrathalamic limit and its organizer properties by regulating Shh induction in the alar plate. J Neurosci 33(9): 3967-3980.
- Vieira, C. and Martinez, S. (2005). Experimental study of MAP kinase phosphatase-3 (Mkp3) expression in the chick neural tube in relation to Fgf8 activity. Brain Res Brain Res Rev 49(2): 158-166.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Martinez-Ferre, A., Navarro-Garberí, M., Bueno, C. and Martinez, S. (2013). Implantation of Dkk-1-soaked Beads into the Neural Tube of Chicken Embryos. Bio-protocol 3(21): e963. DOI: 10.21769/BioProtoc.963.
- Martinez-Ferre, A., Navarro-Garberi, M., Bueno, C. and Martinez, S. (2013). Wnt signal specifies the intrathalamic limit and its organizer properties by regulating Shh induction in the alar plate. J Neurosci 33(9): 3967-3980.
Category
Neuroscience > Development > Neuron
Developmental Biology > Morphogenesis
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









