- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Batch Culture Fermentation of Endophytic Fungi and Extraction of Their Metabolites
Published: Vol 3, Iss 19, Oct 5, 2013 DOI: 10.21769/BioProtoc.926 Views: 15665
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Yeast Single-cell RNA-seq, Cell by Cell and Step by Step
Mariona Nadal-Ribelles [...] Lars M. Steinmetz
Sep 5, 2019 7719 Views

A Microfluidic Platform for Tracking Individual Cell Dynamics during an Unperturbed Nutrients Exhaustion
Théo Aspert [...] Gilles Charvin
Jul 20, 2022 2898 Views
Abstract
Antibiosis is one of the possible modes of action shown by endophytic fungi having antifungal activity. To test if antifungal activity in endophytic fungi is due to antibiosis, assay of the metabolites of endophytic fungi was needed. To obtain metabolites for bioassay batch culture fermentation and extraction of metabolites was done. Fungus was multiplied on wickerham media at incubation temperature of 25 ± 2 °C for 4 weeks and then extracted with solvents of different polarity. All the solvent extracts were dried under vacuum rotary evaporator to get dried crude fungal extract, which was subjected to further fractionation and bioassay.
Keywords: FermentationMaterials and Reagents
- Ethyl acetate (Rankem)
- Butanol (Qualigens)
- Methanol (Qualigens)
- Hexane (Qualigens)
- Fungus culture
- Measuring cylinder
- Whatman filter paper
- Detergent
- Glass jars of 5 L capacity
- Malt extract (HiMedia Laboratories)
- Yeast extract (HiMedia Laboratories)
- Peptone (HiMedia Laboratories)
- Glucose (Qualigens)
- Needle
- Spirit lamp
- Commercial disinfectant (70% ethanol)
- Vacuum pump
- Malt extract
- Yeast extract
- Wickerham medium (see Recipes)
Equipment
- Inoculation flasks
- Conical flasks of 1 L capacity
- Vacuum rotary evaporator (Heidolph Instruments GmbH)
- pH meter (Eutech Instruments pH tutor)
- Autoclave (Nat steel)
- BOD incubator (Toshiba)
- Laminar air flow hood (Toshiba)
- Filteration assembly
- Hand blender (inalsaappliances.com)
- Fume hood
- Microbalances (Sartorious, model: RC210P )
Procedure
- 5 discs of 5 mm diameter of endophytic fungus from petridish were inoculated in Wickerham medium.
- Flasks with inoculated media were incubated at 24 °C for 24 days under static culture condition without light.
- One flask of medium without any inoculam served as a control.
- After 24 days of incubation, 250 ml of ethyl acetate was added to each flask, mixed, and left overnight.
- Ethyl acetate immersed fungus culture was blended with a hand blender for 15 min and filtered by whatman filter paper under vacuum (Wicklow et al., 1998).
- The filtrate was collected and residual aqueous phase was partitioned thrice times with equal volumes of ethyl acetate in a separator funnel (Figure 1).
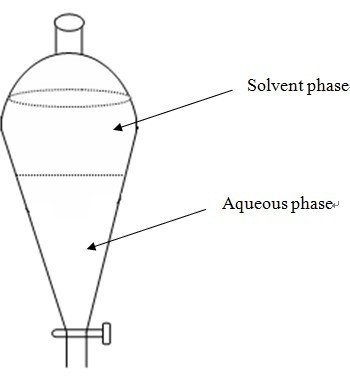
Figure 1. Partitioning by separator funnel - Aqueous phase obtained after ethyl acetate extraction was further partitioned three times with equal volumes of saturated butanol.
- Aqueous phase obtained after butanol extraction was discarded after immersing in detergent.
- The ethyl acetate and butanol extracts were dried with vacuum rotary evaporator.
- Dried ethyl acetate extract resuspended in 90% methanol and extracted with n-hexane.
- After drying the hexane, butanol, and methanol extracts with vacuum rotary evaporator they were subjected to further experimentation. Schematic diagram for the extraction of the metabolite has been given in Figure 2.
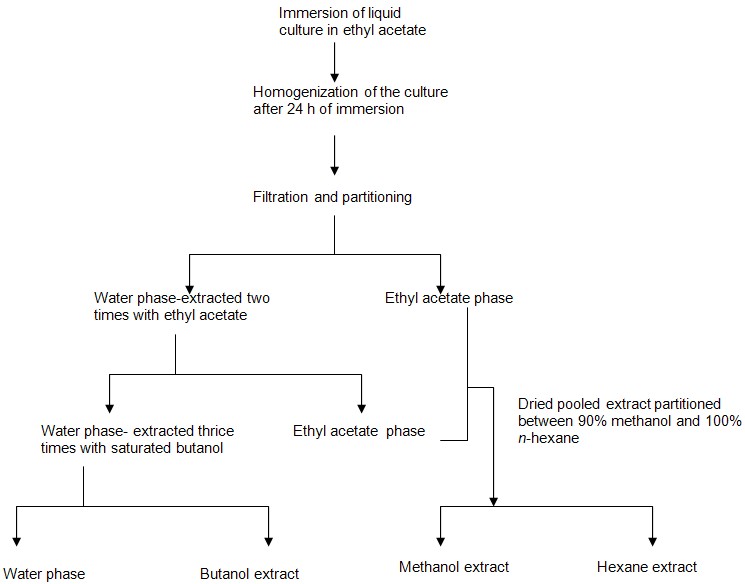
Figure 2. Schematic diagram of extraction procedure for obtaining crude fungal extracts
Recipes
- Wickerham medium
Malt extract 3 g/L
Yeast extract 3 g/L
Peptone 5 g/L
Glucose 10 g/L
All the media chemicals were weighed and dissolved in distilled water and pH was measured. After adjusting the pH in range of 7.2-7.4, media was distributed in conical flasks (300 ml in 1 L conical flask). These flasks were subjected to autoclaving at 121 °C temperature and 15 psi pressure for 20 min.
Acknowledgments
This protocol was adopted from Kumar and Kaushik (2013). Authors are grateful to their host institution, The Energy and Resources Institute (TERI), New Delhi, India for funding the research. Susheel Kumar is grateful to University Grant Commission, New Delhi for a research fellowship.
References
- Kumar, S. and Kaushik, N. (2013). Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One 8(2): e56202.
- Wicklow, D. T., Joshi, B. K., Gamble, W. R., Gloer, J. B. and Dowd, P. F. (1998). Antifungal metabolites (monorden, monocillin IV, and cerebrosides) from Humicola fuscoatra traaen NRRL 22980, a mycoparasite of Aspergillus flavus sclerotia. Appl Environ Microbiol 64(11): 4482-4484.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kumar, S. and Kaushik, N. (2013). Batch Culture Fermentation of Endophytic Fungi and Extraction of Their Metabolites. Bio-protocol 3(19): e926. DOI: 10.21769/BioProtoc.926.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Biochemistry > Other compound > Antimicrobial
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












