- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Virus Overlay Assay (Far-Western blotting)
Published: Vol 3, Iss 19, Oct 5, 2013 DOI: 10.21769/BioProtoc.923 Views: 16070
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

HIV-1 Virus-like Particle Budding Assay
Nathan H Vande Burgt [...] Paul Bates
Jul 20, 2013 11130 Views

Detection of HBV C Protein Phosphorylation in the Cell
Jaesung Jung and Kyongmin Kim
Aug 5, 2015 8586 Views

Coupling of HIV-1 gp120-derived Core Protein to Paramagnetic Beads and Adsorption Assays
Jidnyasa Ingale and Richard T Wyatt
Oct 5, 2015 7336 Views
Abstract
Virus overlay assay is a method to detect protein-protein interaction in vitro. We performed the virus overlay assay to identify the receptor proteins interacting with the infectious spleen and kidney necrosis virus (ISKNV).
Keywords: Far-Western blottingMaterials and Reagents
- Purified ISKNV (was purified in our laboratory)
- 12% SDS-PAGE gel
- PVDF membrane (Whatman, catalog number: 10485290 )
- Coomassie brilliant blue G-250 (Beyotime Institute of Biotechnology, catalog number: P0017B )
- Tris-HCl (pH 7.4)
- EDTA
- Tween 20
- Guanidine hydrochloride (Sigma-Aldrich, catalog number: G4505 )
- Nonfat milk powder
- Maltose-binding protein (MBP) or MBP-mCav-1 protein (these proteins were made in our laboratory)
- Anti-MBP antibodies (Sigma-Aldrich, catalog number: M1321 )
- Horse radish peroxidase (HRP)-conjugated goat anti-mouse secondary antibodies (Invitrogen, catalog number: 626520 )
- HRP substrate solution (Beyotime Institute of Biotechnology, catalog number: P0209 )
- BCA protein assay kit (Thermo Fisher Scientific, catalog number: 23225 )
- Renaturing buffer (see Recipes)
- Tris Buffered Saline and Tween 20 (TBST) (see Recipes)
Equipment
- Electrophoresis equipment
- Bio-Rad blotting equipment (Bio-Rad Laboratories, model: A101441 )
Procedure
- The protein concentrations of the purified virus are determined using the BCA protein assay kit. 100 micrograms of the sample are boiled in 2x SDS-PAGE sample loading buffer for 5 min.
- 30 μl (100 micrograms) denatured product is resolved in two parallel 12% SDS-PAGE gels.
- After electrophoresis, the viral structural proteins in one gel are transferred to a PVDF membrane by electroblotting using the Bio-Rad wet transfer Blotter (50 V for 2 h), whereas the other gel is stained with Coomassie brilliant blue G-250 for a parallel experiment.
- The membrane is washed twice in TBST at room temperature (RT) under constant rotation for 5 min each.
- The blots are then blocked overnight in renaturing buffer at 4 °C, and then incubated with 10 μg/ml MBP or MBP-mCav-1 protein for 2 h at RT.
- The membrane is incubated with anti-MBP antibodies (1:2,000 dilution) in TBST for 2 h at RT after washing three times in TBST under constant rotation for 10 min each.
- The membrane is washed as described in step 4, and the antigen-antibody complexes are then detected using HRP-conjugated goat anti-mouse secondary antibodies (1:5,000 dilution) in TBST for 1 h under constant rotation.
- The membrane is washed as described in step 4, the immobilized conjugates on the membrane are subsequently visualized using an HRP substrate solution. When the member reaches desired intensity, pour off the HRP substrate solution and rinse in several changes of distilled water. Incubate in ddH2O for ~20 min, see an example of the results in Figure 1.
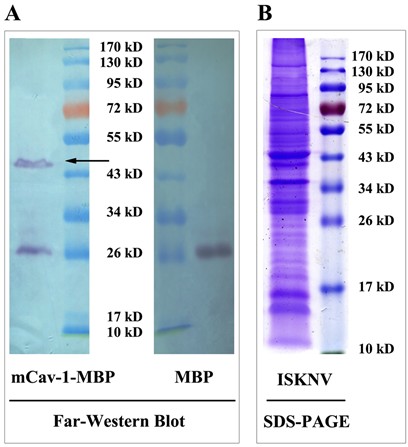
Figure 1. A. Far-Western blot analysis; B. Purified ISKNV particles (100 μg) were isolated using SDS-PAGE, and then stained with Coomassie brilliant blue R-250.
Recipes
- Renaturing buffer
20 mM Tris-HCl
1 mM EDTA
0.5 mol/L NaCl
0.05% Tween 20
1 M guanidine hydrochloride
5% nonfat milk powder
- TBST (1 L)
NaCl 8 g
KCl 0.2 g
Tris base 3 g
Mix in ~ 800 ml dH2O, adjust pH to 7.4 with HCl, then adjust volume to 1 L
Autoclave
After cooling, add 1 ml Tween 20
Acknowledgments
This protocol is adapted from Kikkert et al. (1998).
References
- Kikkert, M., Meurs, C., van de Wetering, F., Dorfmuller, S., Peters, D., Kormelink, R. and Goldbach, R. (1998). Binding of tomato spotted wilt virus to a 94-kDa thrips protein. Phytopathology 88(1): 63-69.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jia, K., Guo, C., Yang, X. and He, J. (2013). Virus Overlay Assay (Far-Western blotting). Bio-protocol 3(19): e923. DOI: 10.21769/BioProtoc.923.
Category
Microbiology > Microbial biochemistry > Protein > Immunodetection
Biochemistry > Protein > Immunodetection > Western blot
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









