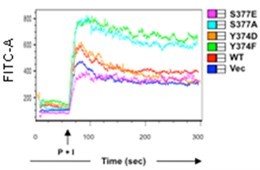
Calcium influx is one of the key signaling events upon stimulation of T cell receptors (TCR) and plays an important role for T cell activation, proliferation, and differentiation. Phorbol myristate acetate (PMA) and calcium ionophore ionomycin are commonly utilized as stimulants in a variety of immunologic assays including T cell activation. PMA is a protein kinase C (PKC) activator, resulting in the activation of Ras, a small GPTase. When PMA and ionomycin are used together, TCR signaling downstream of PKC and Ras can be activated without activation of TCR-triggerd signaling events. This protocol describes the flow cytometry analysis of intracellular calcium influx in T cells stimulated with PMA and ionomycin.