- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Three-dimensional Invasion Assay
Published: Vol 3, Iss 17, Sep 5, 2013 DOI: 10.21769/BioProtoc.885 Views: 13031
Reviewed by: Lin FangFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Studying Chemotactic Migration in Dunn Chamber: An Example Applied to Adherent Cancer Cells
Khedidja Benseddik and Kossay Zaoui
Feb 5, 2022 2945 Views

Spherical Invasion Assay: A Novel Method to Measure Invasion of Cancer Cells
Stephen D. Richbart [...] Piyali Dasgupta
Feb 20, 2022 5421 Views

An Experimental Protocol for the Boyden Chamber Invasion Assay With Absorbance Readout
Kathleen C. Brown [...] Piyali Dasgupta
Aug 5, 2024 2768 Views
Abstract
The invasive ability of cancer cells is a crucial function for cancer metastasis and the surrounding microenvironment of cancer cells in living tissues is three-dimension (3D). Therefore, to establish an in vitro invasion assay in a 3D system to predict cancer invasive ability is valuable in the research for cancer metastasis. Here, we describe a 3D invasion assay for observing the morphology and comparing the invasive ability of cancer cells in artificial 3D environments (Yang et al., 2012). Collagen I gels are used to cover on the top of cancer cells attached on coverslip glass dish and medium containing FBS is added as a chemoattractant. After incubation for a suitable time, the cells are fixed and stained. The invasion index can be calculated and the morphology can be imaged with a laser confocal microscope.
Materials and Reagents
- Cell lines: OECM-1(Huang et al., 2004) and FaDu (ATCC® HTB-43 TM)
- 0.1% Trypsin-EDTA (Life Technologies, Gibco®, catalog number: 15400 )
- 0.1 mg/ml poly-L lysine (Sigma-Aldrich, catalog number: P9404-25MG )
- PBS
- FBS (Thermo Fisher Scientific, catalog number: SH30071.03 )
- PureCor® bovine collagen solution (Advance Biomatrix Inc., catalog number: 5005-B )
- 1 M NaOH solution
- 5x RPMI medium
- 3% Paraformaldehyde (Sigma-Aldrich, catalog number: P6148-500G )
- 0.5% Triton X100 (Bionovas, catalog number: 56-81-5 )
- Alexa Fluor® 488 Phalloidin (Life Technologies, catalog number: A12379 )
- DAPI (Sigma-Aldrich, catalog number: D8417 )
- 1% BSA in PBS
- 1.8 mg/ml collagen I mix solution (see Recipes)
Equipment
- Lab-Tek® chambered #1.0 coverglass system (NUNC, catalog number: 155383 )
- Laser confocal microscope with 60x oil lens (Olympus, model: FV1000 )
- CO2 incubator
Software
- Olympus FV10-ASW 1.7 software
Procedure
Day 1
- Treat Lab-Tek® chambered #1.0 coverglass system with 300 μl of 0.1 mg/ml poly-L lysine solution for one hour at 37 °C.
- Aspirate the poly-L lysine solution and wash one time with PBS.
- Trypsinize cells and 2 x 105 cells in 500 μl medium were plated on coverglass system for attachment.
- After attachment time for 3 to 6 h, prepare the appropriate volume of collagen I mix solution (final concentration 1.8 mg/ml) on ice then carefully remove the medium from coverglass system (avoid to wash cells again) and add 500 μl of collagen I mix solution to coverglass system.
- Cells were incubated at 37 °C, 5% CO2 for 2 h.
- Overlay with 400 μl of medium containing with 15% FBS on collagen gels.
- Incubate at 37 °C, 5% CO2 for 24 to 48 h.
Day 2 or 3
- Carefully aspirate medium from wells and rinse wells including collagen gel invaded by cells with PBS once.
- Carefully pour 400 μl of 3% paraformaldehyde in PBS for 40 min at RT to fix cells.
- Carefully rinse two times with 400 μl PBS.
- Permeabilization in 400 μl of 0.5% Triton X-100 in PBS for 40 min at RT.
- Carefully rinse two times with 400 μl PBS.
- Incubate cells with 400 μl of 1% BSA in PBS for 40 min at RT.
- Stain cells with 500 μl of Alexa Fluor® 488 Phalloidin diluted to 1 units/ml in PBS for 90 min at RT.
- Carefully rinse two times with PBS.
- Stain cells with 500 μl of 2 μg/ml DAPI in PBS for 30 min.
- Wash three times with 400 μl PBS and aspirate all PBS.
- Samples can be stored at 4 °C for 2 weeks or ready to be imaged by a laser confocal microscope. Imaging and quantification.
- Use an Olympus FV1000 laser confocal microscope with 60x oil lens to capture images. The volume of observation is xyz = 210 x 210 x 50 μm3.
- Confocal Z slices are collected each well at 50 μm from the bottom of the well and z interval is set to 1 μm.
- Images of sequential Z sections were obtained and reconstructed by Olympus FV10-ASW 1.7 software.
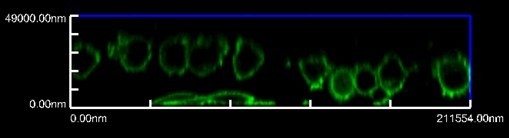
Figure 1. Representative image of FaDu overexpressing Twist1 cells that invaded into collagen after 24 h (please refer to Reference 1 for the detail)
- The invasion index is quantified as the number of cells existing at the distance from the bottom of slide between 30 to 50 μm divided by the total number of cells.
Note: The cells that are partially fallen into the range of 30-50 μm can also be counted.
- The data are presented as the percentage of the invasion index of the control sample and representative vertical sections.
Recipes
- 1.8 mg/ml Collagen I mix solution
1.7 ml PureCor® bovine collagen solution (3 mg/ml)
0.6 ml 5x RPMI
18 μl 1 M NaOH
Add dH2O to 3 ml
All buffers must be on ice before polymerization in the tissue culture incubator. This mix solution must be prepared freshly to use.
Acknowledgments
This protocol was carried out as described in Sanz-Moreno et al. (2008) with minor modifications. The establishment of this protocol was funded by the National Health Research Institutes (NHRI-EX100-10037BI to M-H.Y.; NHRI-EX100-9931BI to K-J.W.); the National Science Council (NSC 99-2314-B-010-007-MY3 and 100-2321-B-010-015 to M-H.Y.; NSC 100-2321-B-039-002 to M-C.H.); Taipei Veterans General Hospital (VGH 100-C-088, 101-C-005 to M-H.Y.); Veterans General Hospitals University System of Taiwan Joint Research Program (VGHUST101-G7-4-1 to M-H.Y.); a grant from the Ministry of Education, Aim for the Top University Plan (to M-H.Y.); a grant from the Department of Health, Center of Excellence for Cancer Research (DOH100-TD-C-111-007 to M-H.Y; DOH101-TD-C-111-005 to M-C.H.); and the Sister Institution Fund of China Medical University and Hospital and MD Anderson Cancer Center.
References
- Huang, G. C., Liu, S. Y., Lin, M. H., Kuo, Y. Y. and Liu, Y. C. (2004). The synergistic cytotoxicity of cisplatin and taxol in killing oral squamous cell carcinoma. Jpn J Clin Oncol 34(9): 499-504.
- Yang, W. H., Lan, H. Y., Huang, C. H., Tai, S. K., Tzeng, C. H., Kao, S. Y., Wu, K. J., Hung, M. C. and Yang, M. H. (2012). RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol 14(4): 366-374.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yang, W. and Yang, M. (2013). Three-dimensional Invasion Assay. Bio-protocol 3(17): e885. DOI: 10.21769/BioProtoc.885.
Category
Cancer Biology > General technique > Tumor microenvironment > Cell migration
Cancer Biology > Invasion & metastasis > Cell biology assays > Cell invasion
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link