- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Seed Germination and Viability Test in Tetrazolium (TZ) Assay
Published: Vol 3, Iss 17, Sep 5, 2013 DOI: 10.21769/BioProtoc.884 Views: 67250
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1954 Views

Closed Systems to Study Plant–Filamentous Fungi Associations: Emphasis on Microscopic Analyses
Vasiliki Skiada and Kalliope K. Papadopoulou
Feb 20, 2025 2865 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views
Abstract
Tetrazolium (TZ) assay is the fast evaluation for seed viability and alternative quick method for seed’s germinability (Porter et al., 1947; Wharton, 1955). All respiring tissues are capable of converting a colourless compound, TZ (2,3,5 triphenyl tetrazolium chloride) to a carmine red coloured water-insoluble formazan by hydrogen transfer reaction catalysed by the cellular dehydrogenases. TZ enters both living and dead cells but only living cells catalyse the formation of formazan which being non-diffusible stains the viable seeds red whereas the absence of respiration prevents formazan production making the dead seeds (aged tissue) remain unstained.
The brief description of this protocol has been reported in Verma et al., 2013.
Materials and Reagents
- Arabidopsis thaliana (Columbia-0) seeds were used in our study
- 2,3,5 triphenyl tetrazolium chloride (Sigma-Aldrich, catalog number: T8877 )
- Commercial bleach (Sodium hypochlorite)
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Autoclaved distilled water
- Lactic acid
- Phenol
- Glycerine
- Whatman no.1 filter paper
- 1% Tetrazolium (TZ) solution (see Recipes)
- Scarification solution (see Recipes)
- Clearing agent (lactophenol solution) (see Recipes)
Equipment
- 30 °C incubator
- Shaker at RT
- Weighing balance
- pH meter
- Stereo Microscope
- Culture room (22 °C ± 2, 16 h light 200 μmol/m2/s/8 h dark)
(any instrument which provides above mentioned conditions will work)
Procedure
- Scarify the Arabidopsis seeds by soaking approximately 100 seeds (in three replicates) in 1 ml scarification solution for 15 min under shaking conditions at RT. Wash at least five times with distilled water to remove the bleach.
Note: Perform steps 1-3 under sterile conditions.
- After scarification, remove excess water and incubate the seeds with 1% TZ solution at 30 °C for 24 to 48 h in dark (observe the seeds after 24 h and proceed with step 3 if stain appears). Take heat killed (100 °C, 1 h) seeds as negative control.
- After staining, wash the seeds 2-3 times with distilled water.
- Immerse the stained seeds in clearing agent for 1-2 h.
Follow step 4, if the pigment within the seed coat prevents clear vision after staining.
- Observe the seeds under stereo microscope.
- Evaluate the seeds on the basis of staining pattern and colour intensity. Among stained seeds, seeds with bright red staining are completely viable while partially stained seeds may produce either normal or abnormal seedlings. Pink or greyish red stain indicates dead tissue. Completely unstained seeds are non-viable. Figure 1 shows TZ staining pattern in viable (normal), abnormal and dead or non-viable seeds.
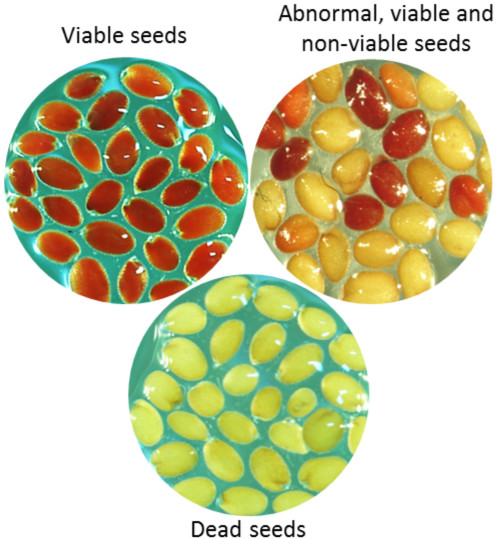
Figure 1. Different pattern of TZ staining showing visable, abnormal and dead or non-viable seeds
- In parallel, place about 100 seeds without scarification on two layers of Whatman no.1 filter paper soaked with distilled water in culture room for germination assay (perform the germination assay in triplicate). Score the seed germination by considering radicle protrusion beyond testa as germinated seeds. To score the germination, count the no. of seeds with radicle protrusion as germinated seeds and calculate the percentage germination against total no. of seeds.
Recipes
- 1% Tetrazolium (TZ) solution
Add 1 g 2,3,5 triphenyl tetrazolium chloride in 100 ml autoclaved distilled water in amber colour bottle.
Mix well and store in dark at 4 °C (can be kept for several months under such conditions).
- Scarification solution
20 ml commercial bleach and 100 μl Triton X-100 in 100 ml autoclaved distilled water. Mix well and store under sterilized conditions (prepare fresh).
- Clearing agent (lactophenol solution)
Mix lactic acid: phenol: glycerine: water in a ratio of 1:1:2:1. Use if the pigment within the seed coat prevents clear vision after staining (prepare fresh).
Notes
- The pH of the TZ staining solution should be 7. Solution with pH > 8 or pH < 4 would result in either intense staining or would not stain even viable seed tissues. If water is out of neutral range then use phosphate buffer with pH 7 to dissolve TZ.
- TZ assay can be used for seeds of legume, cotton and grasses. The incubation time varies with seed type and morphology. Remove the seed coats of larger seeds (like legume seeds) before examination.
- The dicot seeds can be germinated further as the stained seeds are not damaged.
- When performed appropriately, the percentage of viable seeds obtained by tetrazolium assay is very close to the percentage of seed germination expected under most favourable conditions.
Acknowledgments
This protocol was adapted from Porter et al. (1947) and Wharton (1955). This work was supported by the Department of Biotechnology (grant no. BT/PR10262/GBD/27/77/2007) and National Institute of Plant Genome Research, Government of India.
References
- Porter, R., Durrell, M. and Romm, H. (1947). The use of 2, 3, 5-triphenyl-tetrazoliumchloride as a measure of seed germinability. Plant Physiol 22(2): 149.
- Verma, P., Kaur, H., Petla, B. P., Rao, V., Saxena, S. C. and Majee, M. (2013). PROTEIN L-ISOASPARTYL METHYLTRANSFERASE2 is differentially expressed in chickpea and enhances seed vigor and longevity by reducing abnormal isoaspartyl accumulation predominantly in seed nuclear proteins. Plant Physiol 161(3): 1141-1157.
- Wharton, M. J. (1955). The use of tetrazolium test for determining the viability of seeds of the genus Brassica. Proc Int Seed Test Assoc 20: 81-88.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Verma, P. and Majee, M. (2013). Seed Germination and Viability Test in Tetrazolium (TZ) Assay. Bio-protocol 3(17): e884. DOI: 10.21769/BioProtoc.884.
- Verma, P., Kaur, H., Petla, B. P., Rao, V., Saxena, S. C. and Majee, M. (2013). PROTEIN L-ISOASPARTYL METHYLTRANSFERASE2 is differentially expressed in chickpea and enhances seed vigor and longevity by reducing abnormal isoaspartyl accumulation predominantly in seed nuclear proteins. Plant Physiol 161(3): 1141-1157.
Category
Plant Science > Plant cell biology > Tissue analysis
Plant Science > Plant physiology > Plant growth
Plant Science > Plant physiology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Can TTC. Test substitute the germination test, explain with...
Share
Bluesky
X
Copy link












