- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Primary Culture of SVZ-derived Progenitors Grown as Neurospheres
Published: Vol 3, Iss 16, Aug 20, 2013 DOI: 10.21769/BioProtoc.868 Views: 12816
Reviewed by: Xuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs
Louise Thiry [...] Stefano Stifani
Jul 5, 2025 2289 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
SVZ-derived progenitors grown as neurospheres is a well-known model to study neural stem cell and progenitor functions such as proliferation, differentiation/self-renewal, and migration (Durbec and Rougon, 2001). This protocol is for preparing a culture of SVZ-derived progenitors from 8 early postnatal mouse brains (P0 to P3). One week after cell plating, we can observe round floating neurospheres, each resulting from the clonal expansion of a single EGF/FGF responsive neural progenitor.
Keywords: Nervous systemMaterials and Reagents
- New born mice
- Phosphate Buffered Saline (PBS) (Life Technologies, catalog number: 14040-091 )
- Hank's Balanced Salt Solution (HBSS) (Life Technologies, catalog number: 14170-088 )
- Dulbecco's Modified Eagle Medium (DMEM) (Life Technologies, catalog number: 61965-026 )
- Ham’s F-12 nutrient mix (F12) (Life Technologies, catalog number: 31765-027 )
- Trypsin (Sigma-Aldrich, catalog number: T5266 )
- Insulin (Sigma-Aldrich, catalog number: I1882 )
- Holo-transferrin (Sigma-Aldrich, catalog number: T0665 )
- Putrescine (Sigma-Aldrich, catalog number: P5780 )
- Progesterone (Sigma-Aldrich, catalog number: P8783 )
- Sodium selenite (Selenium) (Sigma-Aldrich, catalog number: S5261 )
- Penicillin-streptomycin (Life Technologies, catalog number: 15140-130 )
- Fetal Bovine Serum (FBS) (Life Technologies, catalog number: 10106-169 )
- B27 (Life Technologies, catalog number: 17504-044 )
- rhFGFbasic (Peprotech, catalog number: 167 100-18B-B )
- rhEGF (Peprotech, catalog number: 167 AF-100-15A )
- Neurospheres defined medium (see Recipes)
- Trypsin (see Recipes)
Equipment
- Sterilin Universal Container (Thermo Fisher Scientific, catalog number: 128A/P )
- Standard TC BD Falcon 60 mm cell culture dish (BD Biosciences, Falcon®, catalog number: 353002 )
- Vibratom (Microm, model: HM450 )
- Stereoscopic Microscope
- Cell culture Hood and Incubator
- Scissors, fine forceps, spatula, micro knives
- Ice bucket
- Fire-polished glass Pasteur pipettes
- Water bath
Procedure
- Dissect the brain from new born mice.
- Cut 400 μm thick coronal sections of the brain in ice cold PBS with a Vibratom (Figure 1A) (from the olfactory bulb to the anterior horn of the lateral ventricle). Keep the first two sections of each brain containing the lateral wall of the lateral ventricles and place them in ice cold HBSS.
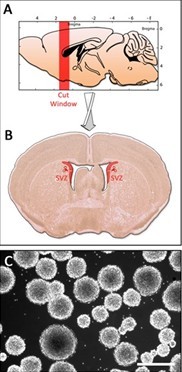
Figure 1. Dissection of the neonatal mouse SubVentricular Zone. A. Illustration of a newborn mice sagittal section showing the cut window when using the vibratome. B. Coronal section showing parts to dissect to isolate lateral ventricules’ tissues. C. Neurospheres obtained after 1 week long culture. Scale bar, 500 μm.
- Under the microscope, dissect out the lateral walls of the lateral ventricles (Figure 1B). Take a forceps with one hand to handle slice and keep it on the bottom of the dish, and a micro knife with the other to cut out the zone of interest. Discard the rest of the slice after each dissection.
- Cut the tissues into small cubes (400 μm cubes) using sterile micro knives.
- Pipette the tissues within a volume of 800 μl HBSS and place them in a 30 ml Sterilin Universal Container. Add 200 μl of trypsin 12.5 mg/ml. Incubate 5 min at 37 °C in a water bath.
- Add 10 volumes of 10% HBSS-FBS. Using fire-polished glass Pasteur pipettes, gently pipet up and down to help the dissociation. Take of a droplet regularly and check under the microscope whether the dissociation is complete.
- Centrifuge at 800 x g for 7 min at room temperature (RT), discard the supernatant, and resuspend the pellet in 10 ml of fresh HBSS. Take of a sample and count the number of cells.
- Centrifuge the cell suspension at 800 x g for 7 min at RT, discard the supernatant, and resuspend in Standard TC BD Falcon 60 mm cell culture dishes at the concentration of 25,000 cells/ml in neurospheres defined medium, supplemented with 2% B27, bFGF (20 ng/ml) and EGF (20 ng/ml).
- Every 3 days, add a half-volume of doubly-supplemented [4% B27, bFGF (40 ng/ml) and EGF (40 ng/ml)] neurospheres defined medium. To avoid any sphere attachment to the bottom of the culture dish, don’t extend the culture beyond 1 week (Figure 1C).
Recipes
- Neurospheres defined medium
DMEM/F12, 3:1 volumes respectively
5 g/ml Insulin
100 g/ml Holo-transferrin
100 M Putrescine
20 nM Progesterone
30 nM Selenium
1% Penicillin-streptomycin (100 IU/ml and 100 g/ml, respectively)
- Trypsin (prepare each time a new aliquot)
Dissolve 12.5 mg of Trypsin in 1 ml of ice cold HBSS
Acknowledgments
This protocol is adapted from Durbec and Rougon (2001) and Vernerey et al. (2013).
References
- Durbec, P. and Rougon, G. (2001). Transplantation of mammalian olfactory progenitors into chick hosts reveals migration and differentiation potentials dependent on cell commitment. Mol Cell Neurosci 17(3): 561-576.
- Vernerey, J., Macchi, M., Magalon, K., Cayre, M. and Durbec, P. (2013). Ciliary neurotrophic factor controls progenitor migration during remyelination in the adult rodent brain. J Neurosci 33(7): 3240-3250.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Vernerey, J., Magalon, K. and Durbec, P. (2013). Primary Culture of SVZ-derived Progenitors Grown as Neurospheres. Bio-protocol 3(16): e868. DOI: 10.21769/BioProtoc.868.
- Vernerey, J., Macchi, M., Magalon, K., Cayre, M. and Durbec, P. (2013). Ciliary neurotrophic factor controls progenitor migration during remyelination in the adult rodent brain. J Neurosci 33(7): 3240-3250.
Category
Neuroscience > Development > Neuron
Stem Cell > Adult stem cell > Neural stem cell
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link