- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Growth Cones from Mouse Brain
Published: Vol 3, Iss 15, Aug 5, 2013 DOI: 10.21769/BioProtoc.853 Views: 11893
Reviewed by: Xuecai Ge

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3773 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2728 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2636 Views
Abstract
Growth cones are motile structures at the tips of growing neurites, which play an essential role in regulation of growth and navigation of growing axons and dendrites of neurons in the developing nervous system. This protocol describes isolation of growth cones from the brain tissue from young mice. Growth cones isolated using this protocol have been extensively characterized using electron microscopy (Pfenninger et al., 1983) and may be used for any kind of subsequent biochemical and/or functional analyses, including Western blot analysis of protein expression (Westphal et al., 2010), analysis of the activity of growth cone-accumulated enzymes (Leshchyns’ka et al., 2003; Li et al., 2013), and analysis of the endocytosis and exocytosis rates (Chernyshova et al., 2011).
Keywords: NeuronsMaterials and Reagents
- Mouse brains extracted from 1-3 days old mice, frozen in liquid nitrogen and kept at -80 °C (for up to 1 year)
- Sucrose
- Purified (e.g. using Milli-Q system from Millipore) water kept at 4 °C
- Mini EDTA-free Protease Inhibitor Cocktail Tablets (Roche Applied Science, catalog number: 05892791001 )
- PMSF (Sigma-Aldrich, catalog number: P7626 )
- Ethanol
- 80% sucrose (see Recipes)
- Homogenization buffer (see Recipes)
- 0.75 M sucrose buffer (see Recipes)
- 1 M sucrose buffer (see Recipes)
- 2.33 M sucrose buffer (see Recipes)
Equipment
- Potter homogenizer (Thermo Fisher Scientific, catalog number: 08-414-14A )
- 1 ml plastic pipette (Sarstedt, model: 86.1180 )
- Bench top centrifuge with an angle rotor, for example Allegra X-15R (Beckman Coulter)
- Ultracentrifuge with a swing rotor, for example L-60 ultracentrifuge with SW40Ti rotor (Beckman) or HIMAC CP100WX ultracentrifuge with P40ST rotor (Hitachi)
- Centrifuge tube 13PA (Hitachi, catalog number: 332901A )
Procedure
- Prepare 80% sucrose in advance and keep it at 4 °C.
- Prepare buffers for homogenization and centrifugation immediately before preparation of growth cones and place them on ice.
- Take 10 brains from the -80 °C freezer and place them on ice. Proceed immediately to the next step.
- Transfer brains to the Potter homogenizer and add homogenization buffer. Use 1 ml of buffer for homogenization per 1 brain.
- Homogenize brains.
Note: If you isolate growth cones from several experimental groups, use the same numbers of strokes to homogenize brains in each group. - Centrifuge homogenate at 1,660 x g for 15 min at 4 °C using the bench top centrifuge.
- Collect the supernatant.
- Prepare a discontinuous 0.75/1.0/2.33 M sucrose density gradient. To prepare the gradient, carefully pipette into a centrifuge tube 1 ml of ice cold 2.33 M sucrose buffer (bottom), then 3 ml of ice cold 1.0 M sucrose buffer, and then 4 ml of ice cold 0.75 M (top) sucrose buffer. Volumes are given for the centrifuge tube 13PA. To prevent intermixing of the sucrose layers during the gradient preparation, tilt the tube and place the tip of the pipette against the wall of the tube at the top of the tube (Figure 1). Release the solutions to the tube slowly.
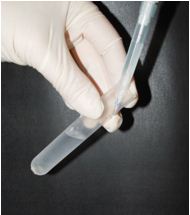
Figure 1. Position of the centrifuge tube and pipette during formation of a sucrose gradient - Load the supernatant on top of the gradient and centrifuge at 242,000 x g for 60 min at 4 °C.
- Collect the growth cone enriched fraction at the interface between the load and 0.75 M sucrose (Figure 2A). The growth cone depleted fraction can be collected between 0.75 M and 1.0 M sucrose (Figure 2A) and may be used as non-growth cone membranes. To collect the growth cone-enriched fraction, squeeze the bulb of a 1 ml plastic pipette, then carefully place the tip of the pipette into the layer of the sucrose gradient containing growth cones (Figure 2B), and then slowly release the bulb of the pipette to allow the growth cone-containing solution to flow into the pipette. Then carefully remove the pipette containing growth cones from the centrifuge tube and release the growth cone fraction into a clean centrifuge tube. Repeat if required.
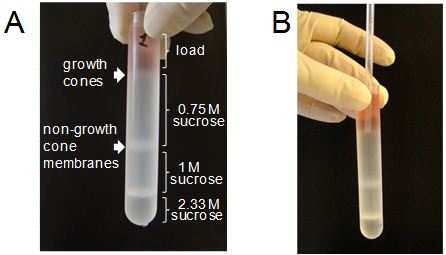
Figure 2. Collection of the growth cone enriched fraction from a sucrose gradient. A: Distribution of the sucrose layers and the interfaces containing fractions enriched in growth cones and non-growth cone membranes in the centrifuge tube after the centrifugation. B: Position of the tip of a plastic pipette during collection of the growth cone-enriched fraction.
- Re-suspend the growth cone fraction in the buffer for homogenization by adding this buffer to the tube to fill it to capacity. Centrifuge at 100,000 x g for 40 min at 4 °C.
- Collect the pellet containing growth cones, re-suspend it in 50 μl of the homogenization buffer and freeze at -80 °C. Important, the homogenization buffer has to contain protease inhibitors as described in the section Recipes. Growth cones thus obtained can be stored at -80 °C for up to 1 week for Western blot analysis. Growth cones for functional analyses (e.g. analysis of exo- and endocytosis) have to be used immediately and cannot be frozen.
Note: To check the growth cone isolation efficiency, the growth cone enriched fraction can be analyzed by Western blot. The growth cone fraction have to be enriched in growth-associated protein (GAP-43) and the neural cell adhesion molecule (NCAM) when compared to brain homogenates and non-growth cone membranes. Non-growth cone membranes, which also contain Golgi membranes, have to be enriched in Golgi matrix protein GM130, while the growth cone fraction should have only low levels of this protein due to the presence of Golgi-derived vesicles in growth cones.
Recipes
- 80% sucrose
For 500 ml of the solution
Weigh 400 g of sucrose in a clean 500 ml Erlenmeyer flask.
Fill the flask with water up to a 500 ml mark.
Put the flask on a magnetic stirrer hotplate and mix with a magnetic stirrer until the sucrose is dissolved. You may heat the solution up to 50 °C to increase the sucrose solubility.
The solution can be stored at 4 °C for up to 1 month. - Homogenization buffer
5 mM Tris-HCl
0.32 M sucrose
1 mM MgCl2
For 100 ml of buffer
13.6 ml of 80% sucrose
0.1 ml of 1 M MgCl2
0.5 ml of 1 M Tris-HCl buffer (pH 7.4)
85.8 ml of water
Mix well and keep on ice. Add protease inhibitors just before usage. Use one tablet of the EDTA-free protease inhibitor cocktail and 10 μl of 1 mM PMSF for 10 ml of buffer. Tablets require some time to be dissolved. - 0.75 M sucrose
For 100 ml of buffer
32 ml of 80% sucrose
0.1 ml of 1 M MgCl2
0.5 ml of 1 M Tris-HCl buffer (pH 7.4)
67.4 ml of water
Mix well and keep on ice. - 1 M sucrose
For 100 ml of buffer
42.7 ml of 80% sucrose
0.1 ml of 1 M MgCl2
0.5 ml of 1 M Tris-HCl buffer (pH 7.4)
56.7 ml of water
Mix well and keep on ice. - 2.33 M sucrose
For 100 ml of buffer
99.4 ml of 80% sucrose
0.1 ml of 1 M MgCl2
0.5 ml of 1 M Tris-HCl buffer (pH 7.4)
Mix well and keep on ice.
References
- Chernyshova, Y., Leshchyns'ka, I., Hsu, S. C., Schachner, M. and Sytnyk, V. (2011). The neural cell adhesion molecule promotes FGFR-dependent phosphorylation and membrane targeting of the exocyst complex to induce exocytosis in growth cones. J Neurosci 31(10): 3522-3535.
- Leshchyns'ka, I., Sytnyk, V., Morrow, J. S. and Schachner, M. (2003). Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol 161(3): 625-639.
- Li, S., Leshchyns'ka, I., Chernyshova, Y., Schachner, M. and Sytnyk, V. (2013). The neural cell adhesion molecule (NCAM) associates with and signals through p21-activated kinase 1 (Pak1). J Neurosci 33(2): 790-803.
- Pfenninger, K. H., Ellis, L., Johnson, M. P., Friedman, L. B. and Somlo, S. (1983). Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell 35(2 Pt 1): 573-584.
- Westphal, D., Sytnyk, V., Schachner, M. and Leshchyns'ka, I. (2010). Clustering of the neural cell adhesion molecule (NCAM) at the neuronal cell surface induces caspase-8- and -3-dependent changes of the spectrin meshwork required for NCAM-mediated neurite outgrowth. J Biol Chem 285(53): 42046-42057.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Leshchyns’ka, I. and Sytnyk, V. (2013). Isolation of Growth Cones from Mouse Brain. Bio-protocol 3(15): e853. DOI: 10.21769/BioProtoc.853.
- Li, S., Leshchyns'ka, I., Chernyshova, Y., Schachner, M. and Sytnyk, V. (2013). The neural cell adhesion molecule (NCAM) associates with and signals through p21-activated kinase 1 (Pak1). J Neurosci 33(2): 790-803.
Category
Neuroscience > Development > Neuron
Cell Biology > Organelle isolation > Fractionation
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











