- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assay of Blood Brain Barrier and Placental Barrier Permeability
(*contributed equally to this work) Published: Vol 3, Iss 15, Aug 5, 2013 DOI: 10.21769/BioProtoc.845 Views: 12673
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3959 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2647 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1262 Views
Abstract
Evans blue dye solution was used to observe the effect of a viral protein on two pre-defined barriers of the body i.e. the blood brain barrier (BBB) and the placental barrier (PB). This dye has strong affinity for serum albumin and does not cross these barriers under natural conditions. As all the dye gets bound to albumin, all the neural tissues and embryonic tissues remain unstained. When the BBB and PB are compromised due to the breach of these barriers, albumin-bound Evans blue enters the CNS and the placenta.
Materials and Reagents
- Pregnant Sprague Dawley rats
- Protein of Interest: Recombinant Nef
- Evans blue dye (Sigma-Aldrich)
- NaCl (Sigma-Aldrich)
- Phosphate buffer saline (PBS) (Sigma-Aldrich)
Equipment
- Homogenizer (Coleparmer)
- Centrifuge (Eppendorf)
- Weighing Balance (Mettler Toledo)
- Spectrophotometer (Gene Quant)
Procedure
- 2% Evans Blue dye was dissolved in normal saline (0.85% sodium chloride). 500 μl of dye containing recombinant protein was injected intravenously in the tail vein of fourteen days pregnant Sprague Dawley rats.
- Recombinant protein in the range 50-500 μg was used to identify the threshold value needed for the breach of both the barriers. Un-injected animals were used for the normalization of the data.
- One hour after inoculation all the rats including the un-injected ones were anaesthetized and dissected immediately to avoid any blood clotting; complete uterus and the brain were removed carefully in normal saline.
- The fetal tissues; uterus, placenta, amniotic membrane and embryo were separated cautiously and collected in PBS to measure the weight of these organs separately.
- Each fetal tissue was measured by weight and then homogenized in 200 μl of PBS (pH 7.4), the final volume was measured again and the mg/ml concentration was calculated.
- The homogenized tissue was centrifuged at ~9,000 x g at 4 °C for 15 min and the clear supernatant was collected.
- The absorbance of Evans blue dye was measured at OD590nm from the brain as well as fetal tissues associated with blood-brain barrier and placental barrier respectively.
- The absorbance (OD) per mg of tissue weight was determined from the supernatant at 590 nm for the quantitative analysis of Evans blue dye.
- Absorbance was considered as an average of four animals and the actual absorbance was calculated after normalizing the background values from the un-injected control set of tissues.
- Figure 1 explains the breach of these blood barriers in the presence of the recombinant protein, whereas no breach was observed in the absence of the recombinant protein.
- If the blood barriers breaches, the absorbance was found to be higher and the dye permeability was observed, whereas if the blood barrier is intact then the absorbance was comparatively lower and no permeability was observed for the dye.
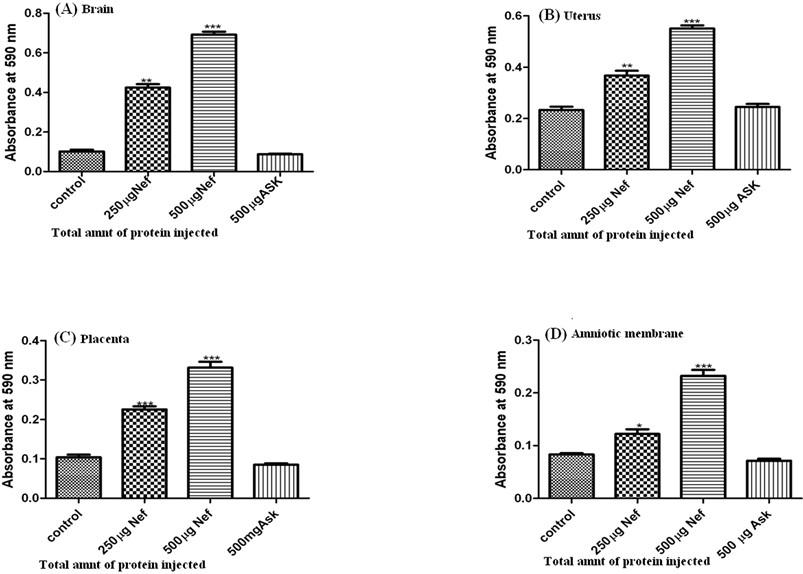
Figure 1. Quantification of Evans blue dye (OD at 590) present (within an hour) in brain and different fetal tissue isolates from 14 day pregnant Sprague Dawley rats injected intravenously without and with recombinant Nef and ASK-1 protein. (A) Brain (B) Uterus (C) Placenta (D) Amniotic membrane. Three different bars in each set represent 0, 250 and 500 μg of recombinant Nef and 500 μg of ASK-1 injected intravenously along with Evans blue dye in the experimental animals. As data represent ±SEM of 3 separate experiments in duplicate and changes were considered as significant at *p ≤ 0.05,**p ≤ 0.01 and ***p ≤ 0.001.
Acknowledgments
This protocol is adapted from Chaturverdi et al. (1991); Singh et al. (2012) and Thumwood et al. (1988).
References
- Chaturvedi, U. C., Dhawan, R., Khanna, M. and Mathur, A. (1991). Breakdown of the blood-brain barrier during dengue virus infection of mice. J Gen Virol 72 ( Pt 4): 859-866.
- Singh, P., Agnihotri, S. K., Tewari, M. C., Kumar, S., Sachdev, M. and Tripathi, R. K. (2012). HIV-1 Nef breaches placental barrier in rat model. PLoS One 7(12): e51518.
- Thumwood, C. M., Hunt, N. H., Clark, I. A. and Cowden, W. B. (1988). Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology 96 ( Pt 3): 579-589.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Agnihotri, S. K., Singh, P., Kumar, B., Singh, P., Jain, S. K., ChandraTewari, M., Kumar, S., Sachdev, M. and Tripathi, R. K. (2013). Assay of Blood Brain Barrier and Placental Barrier Permeability. Bio-protocol 3(15): e845. DOI: 10.21769/BioProtoc.845.
Category
Cell Biology > Cell staining > Protein
Cell Biology > Tissue analysis > Tissue isolation
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










