- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo Neurogenesis
Published: Vol 3, Iss 15, Aug 5, 2013 DOI: 10.21769/BioProtoc.841 Views: 13984
Reviewed by: Lin FangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3773 Views

Derivation and Culture of Enriched Phrenic-Like Motor Neurons From Human iPSCs
Louise Thiry [...] Stefano Stifani
Jul 5, 2025 2289 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2636 Views
Abstract
This protocol shows how to characterize the dynamics of hippocampal neurogenesis in the adult mouse by describing preparation of brain tissue, immunofluorescence of brain sections and confocal stereotactic cell counting.
Materials and Reagents
- Mice
- 5-Bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich, catalog number: B5002 )
- 0.9% sterile sodium chloride (NaCl) (Fresenius Kabi)
- Ketamine hydrochloride (Ketavet, 100 mg/ml) (Pfizer)
- Xylazine hydrochloride (Rompun, 20 mg/ml Xylazine) (Bayer)
- 4% Paraformaldehyde in phosphate buffer (4% Roti-Histofix) (Roth, catalog number: P087.1 )
- Hank’s balanced salt solution (HBSS) (Life Technologies, InvitrogenTM, catalog number: 14170-138 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 31434 )
- Sodium phosphate dibasic heptahydrate (Na2HPO4.7H2O) (Sigma-Aldrich, catalog number: S9390 )
- Sodium phosphate monobasic monohydrate (NaH2PO4.H2O) (Roth, catalog number: K300.2 )
- Potassium chloride (KCl) (AppliChem GmbH, catalog number: A3582 )
- Potassium phosphate monobasic (KH2PO4) (GERBU Biotechnik GmbH, catalog number: 2018 )
- Hydrochloric acid (HCl, 37%) (Sigma-Aldrich, catalog number: 30721 )
- Trizma base (Sigma-Aldrich, catalog number: T1503 )
- Horse serum (Biochrom, catalog number: S9135 )
- Triton X-100 (Sigma-Aldrich, catalog number: X-100 )
- Boric acid (Fluka, catalog number: 15660 )
- Sodium tetraborate decahydrate (Sigma-Aldrich, catalog number: S9640 )
- Hoechst (33342) (dilute with H2O to 10 mg/ml) (Biotrend, catalog number: 40047 )
- Sodium azide (Sigma-Aldrich, catalog number: S2002 )
- Superglue
- Antibodies (see Table 1 and 2)
- Agarose (AppliChem GmbH, catalog number: A8963 )
- Phosphate buffered saline (PBS) (20x) (see Recipes)
- TBS (10x) (see Recipes)
- TBS++ (see Recipes)
- Boric buffer (see Recipes)
- 0.1 M Phosphate buffer (PB) (see Recipes)
- Mowiol (Merck/Calbiochem, catalog number: 475904 ) (see Recipes)
Equipment
- Syringe (1 ml syringe 27 G for i.p. injections)
- 0.5 ml Eppendorf Safelock tubes
- 15 ml and 50 ml Falcon tubes
- Petri dish
- Micro dissecting scissors
- Forceps
- Leica VT1200 Vibratome
- Brush to transfer slices
- Netwell carriers and plates (Corning Incorporated, catalog number: 3477 and 3520 )
- Rocking platform
- Tube roller mixer
- Microscope glass slides
- Cover slips
- Confocal microscope
- Shaker
- Centrifuges
Software
- ImageJ
Procedure
- BrdU administration
- Mice are injected intraperitoneal (i.p.) with 300 mg/kg bodyweight BrdU (15 mg/ml diluted in 0.9% NaCl) and perfused after 24 h to analyze proliferation of neural progenitor cells.
- Alternatively, to study survival and differentiation of new-born cells mice are injected i.p. on three consecutive days with 300 mg/kg bodyweight per day BrdU and perfused 4 weeks after to analyse BrdU-positive cells.
- Transcardial perfusion
- Animals are anesthetized with an overdose of Rompun (14 mg/kg bodyweight) and Ketavet (100 mg/kg bodyweight) in 0.9% NaCl.
- Mice are transcardially perfused with 30 ml HBSS followed by 10 ml of 4% paraformaldehyde (PFA) dissolved in 0.1 M phosphate buffer pH 7. For transcardial perfusion the thorax cavity is opened, and the right auricle cut with a scissor to allow bleeding. A butterfly cannula is introduced into the left ventricle and mice are perfused with 30 ml HBSS followed by fixation with 10 ml 4% PFA.
- Brains are removed and post-fixed overnight in 10 ml 4% PFA in 0.1 M phosphate buffer pH 7 in a 15 ml Falcon tube on a tube roller mixer at 4 °C.
- Tissue is washed twice with PBS and may be stored in PBS with 0.01% sodium azide for up to a year.
- Animals are anesthetized with an overdose of Rompun (14 mg/kg bodyweight) and Ketavet (100 mg/kg bodyweight) in 0.9% NaCl.
- Vibratome cutting
- For coronal vibratome sections (see Figure 1)
- The cerebellum is cut, removed.
- The brain glued upright with the cutting site using superglue onto the holder plate of the vibratome.
- Coronal sections may have a thickness of 50 μm or 100 μm.
Note: The NeuN antibody does not very well penetrate 100 μm thick sections. If you have to use 100 μm thick sections primary antibody incubation should be 72 h.
- The cerebellum is cut, removed.
- For sagittal brain sections (see Figure 1)
- Sagittal brain sections are embedded in 2% agarose in PBS.
- The brain is then glued in a solid gel block on the lateral side to the holder plate.
- Sagittal sections are cut 100 μm thick.
- Agarose can be removed from the slices after cutting or may be kept during the staining process in order to stabilize the tissue (especially olfactory bulbs).
- Sagittal brain sections are embedded in 2% agarose in PBS.
- Sections are stored in PBS with 0.01% sodium azide at 4 °C. Tissue might be used for up to one year after perfusion.

Figure 1. Overview of neurogenic niches in coronal and sagittal brain sections (SVZ: subventricular zone; DG: dentate gyrus)
- For coronal vibratome sections (see Figure 1)
- Immunofluorescence staining
- For each mouse, 6 coronal brain slices (50 μm thick) 250 μm apart are stained, starting from the first slice, when both, upper and lower blade of the DG, are present on the slice. If saggital sections are used, 3 slices from each hemisphere 3 slices (300 μm) apart are used. Brain sections are placed in net carriers in 12 well plates (2 slices per well) filled with 4 ml 0.1 M Tris buffer (pH 7.4) supplemented with 0.8% NaCl (TBS). The sections are washed three times in TBS each 15 min at RT on a rocking platform (50 rpm).
- BrdU staining
- BrdU staining requires DNA denaturization by incubating the brain sections in 2 N HCl (always prepare fresh) at 37 °C on a shaker for 30 min.
- Thereafter, sections are neutralized by washing in Boric buffer for 10 min on a shaker at RT.
- Next, sections are washed six times in TBS for 15 min each at RT.
- BrdU staining requires DNA denaturization by incubating the brain sections in 2 N HCl (always prepare fresh) at 37 °C on a shaker for 30 min.
- Blocking of unspecific antibody binding is performed by incubating sections for 1 h in TBS++ in net carriers at RT.
- Sections are transferred to 0.5 ml Eppendorf Safelock tubes (2 sections per tube) containing 200 μl TBS++ and the diluted primary antibodies, and incubated at 4 °C for 24-72 h. For this 12 tubes are put in a 50 ml Falcon and rotated at 4 °C on a tube roller mixer. A selection of primary antibodies is listed in Table 1.
Table 1. Primary Antibody list
Tbr2 Antibody: Some lots work for paraffin sections, others work for the vibratome sections that are used here (see Figure 2). Ask Abcam for information about that or order 2-3 vials to test different lots.Antibody Host species Manufacturer Catalog number Dilution anti-BrdU rat AbD Serotec OBT0030CX 1:500 anti-cleaved Caspase-3 rabbit Cell Signaling 9661 1:200 anti-DCX (C-18) goat Santa Cruz sc-8066 1:250 anti-GFAP mouse Millipore MAB360 1:400 anti-GFAP rabbit Millipore AB5804 1:400 anti-GFP chicken Aves GFP-1020 1:1,000 anti-NeuN mouse Millipore MAB377 1:200 anti-S100β mouse Sigma-Aldrich S2532 1:200 anti-Sox2 rabbit Abcam ab92494 1:500 anti-Tbr2 (Eomes) rabbit Abcam ab23345 1:500 - After incubation sections are transferred back to net carriers in 12 well plates, washed three times with TBS at RT.
- After blocking in TBS++ for 30 min at RT sections are transferred again into 0.5 ml Eppendorf Safelock tubes containing the diluted secondary antibody mix in TBS++. Sections in Eppdorf tubes in Falcons are incubated in secondary antibodies at 4 °C on a tube roller mixer for 2 h. Secondary antibodies are listed in Table 2. Hoechst 33342 (1:10,000) is used to counterstain DNA and added to the secondary antibody mix.
Table 2. Secondary Antibody listAntibody Manufacturer Catalog number Dilution donkey anti-chicken DyLight488 Dianova 703-485-155 1:400 donkey anti-goat Alexa 488 Invitrogen A-11055 1:400 donkey anti-goat Alexa 456 Invitrogen A11057 1:400 donkey anti-goat Alexa 647 Dianova 705-605-147 1:400 donkey anti-mouse DyLight488 Dianova 715-485-150 1:400 donkey anti-mouse Alexa 546 Invitrogen A10036 1:400 donkey anti-rabbit DyLight488 Dianova 711-485-152 1:400 donkey anti-rabbit DyLight 649 Dianova 711-495-152 1:400 donkey anti-rat Alexa 488 Dianova 712-545-150 1:400 donkey anti-rat rhodamine red Dianova 712-296-150 1:400 - Finally sections are placed back into net carriers in 12 well plates washed three times for 15 min with TBS and additionally 4 times for 1 min in TBS at RT.
- Sections are floated in 0.1 M PB in a Petri dish, mounted on glass slides and embedded with 100 μl Mowiol.
- For each mouse, 6 coronal brain slices (50 μm thick) 250 μm apart are stained, starting from the first slice, when both, upper and lower blade of the DG, are present on the slice. If saggital sections are used, 3 slices from each hemisphere 3 slices (300 μm) apart are used. Brain sections are placed in net carriers in 12 well plates (2 slices per well) filled with 4 ml 0.1 M Tris buffer (pH 7.4) supplemented with 0.8% NaCl (TBS). The sections are washed three times in TBS each 15 min at RT on a rocking platform (50 rpm).
- Confocal microscopy
- Confocal microscope pictures can be taken with a 20x, 40x or 60x objective on a confocal microscope.
- Cell numbers can be counted manually or by using the Cell counter plug in of ImageJ.
- Cell numbers are normalized to the volume of the DG granule cell layer measured by ImageJ.
- Confocal microscope pictures can be taken with a 20x, 40x or 60x objective on a confocal microscope.
Representative data
Not all Tbr2 Eomes antibody (AB) lots work for this protocol. Below are representative pictures and sample lots that show a specific staining (A) of Tbr2 in vibratome sections and antibody lots that give only unspecific background staining (B). Red arrows in A show Tbr2 staining. The green arrows indicate unspecific background staining of glia-like cells that might appear in the specific antibody lots. This background staining is however very well distinguishable from the nuclear Tbr2 staining.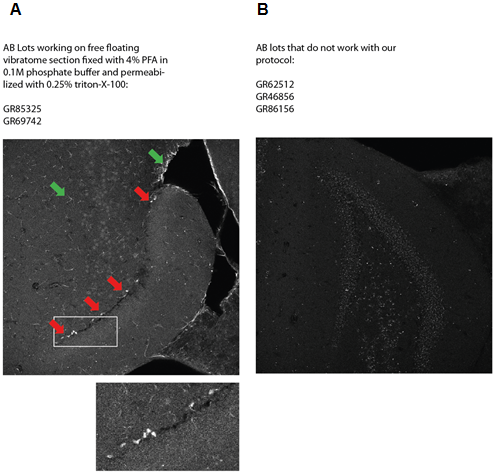
Figure 2. A specific staining (A) of Tbr2 in vibratome sections and antibody lots that give only unspecific background staining (B).
Recipes
- PBS (20x)
Adjust pH to 7.4 with HCl and fill volume up to 1 L with dH2O.NaCl 160 g/L Na2HPO4 23 g/L Na2HPO4 28.84 g/L KCl 4 g/L KH2PO4 4 g/L - TBS (10x)
Mix in 800 ml ultra-pure water, adjust pH to 7.6 with pure HCl and fill up to 1 L.Trizma base 24.23 g/L NaCl 80.06 g/L - TBS ++
TBS 100 ml Horse serum 3 ml Triton X-100 0.25 ml - Boric buffer
Mix in 800 ml ultra pure water, adjust pH to 7.6 and fill up to 1 L.Boric acid 3.1 g/L Sodium tetraborate 4.75 g/L - 0.1 M Phosphate buffer
0.2 M Monobasic Stock
Combine indicated amounts of 0.2 M monobasic and 0.2 M dibasic stock solutions and bring volume up to 600 ml.NaH2PO4.H2O 13.9 g/500 ml 0.2 M Dibasic stock Na2HPO4.7H2O 53.65 g/L 0.2 M Monobasic Stock 0.2 M Dibasic Stock pH 57 ml 243 ml 7.4 - Mowiol
Centrifuge 15 min at 5,000 rpm, RT1x PBS 40 ml Mowiol 10 g → stir for 24 h Add Glycerol 20 ml → stir for 24 h
Aliquot and store at -20 °C
Acknowledgments
This protocol is adapted from Seib et al. (2012).
References
- Seib, D. R., Corsini, N. S., Ellwanger, K., Plaas, C., Mateos, A., Pitzer, C., Niehrs, C., Celikel, T.nd Martin-Villalba, A. (2013). Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 12(2): 204-214.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Seib, D. R. M. and Martin-Villalba, A. (2013). In vivo Neurogenesis. Bio-protocol 3(15): e841. DOI: 10.21769/BioProtoc.841.
Category
Neuroscience > Neuroanatomy and circuitry > Animal model
Stem Cell > Adult stem cell > Neural stem cell
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link