- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transfection of Human Naive CD4+ T Cells with PHA Activation and Neon Electroporation
Published: Vol 3, Iss 15, Aug 5, 2013 DOI: 10.21769/BioProtoc.836 Views: 17880
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

T-Cell-Based Platform for Functional Screening of T-Cell Receptors Identified in Single-Cell RNA Sequencing Data Sets of Tumor-Infiltrating T-Cells
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
Apr 20, 2024 6409 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2728 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3550 Views
Abstract
Transfection of primary T cells can be challenging. This protocol describes a method to transfect primary human naive CD4+ T cells with an AP-1 luciferase reporter using low-level activation by phytohemagglutinin (PHA) and electroporation, as published (Palin et al., 2013). This technique is a modification of one previously described by our group (Cron et al., 2013). Anyone wishing to transfect murine T cells should consult the publication by Cron et al., 2013. This technique may be adapted for other primary T cell types by optimizing the Neon electroporation conditions, as described in the text. Other luciferase or GFP reporters may be used, and will require optimization of the stimulation conditions for that particular reporter.
Keywords: Human CD4+ T cellsMaterials and Reagents
- Human blood or peripheral blood mononuclear cells (PBMCs), collected using heparin (1 ml per 60 ml of blood as an anti-coagulant)
- Heparin (Sigma-Aldrich, catalog number: H3393 )
- Ficoll-Hypaque (GE Life Sciences, catalog number: 17-1440-02 )
- Human MACS Naive CD4+ T cell II Kit (Miltenyi Biotec, catalog number: 130-094-131 )
- BSA (Thermo Fisher Scientific, catalog number: SH30574 )
- PBS (Life Technologies, InvitrogenTM, catalog number: 10010 )
- RPMI medium (Life Technologies, InvitrogenTM, catalog number: 11875093 )
- Heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals or other supplier)
- Hank's balanced salt solution without calcium or magnesium (HBSS) (Life Technologies, InvitrogenTM, catalog number: 14170161 )
- PHA (Sigma-Aldrich, catalog number: 61764 )
- Neon Transfection System 100 μl kit (Life Technologies, InvitrogenTM, catalog number: MPK10096 or MPK10025 )
- Aqua Amine Live/Dead discriminator (Life Technologies, InvitrogenTM, catalog number: L34957 )
- 7AAD (BD Biosciences, catalog number: 559925 )
- Anti-CD3+ anti-CD28- coated Dynal beads (Life Technologies, InvitrogenTM, catalog number: 11131D )
- Ionomycin (Sigma-Aldrich, catalog number: I3909 )
- Phorbol myristate acetate (PMA) (Sigma-Aldrich, catalog number: P8139 )
- One Glo luciferase reagent (Promega Corporation, catalog numbers: E6110 , E6120 , or E6130 )
- AP-1-luciferase reporter (as described in Vaysberg et al., 2008)
Note: This plasmid consists of a pGL3 backbone (Promega Corporation, catalog number: E1751) with 5 copies of the AP-1 binding site from the metallothionein promoter inserted into the human IL2 minimal promoter. - pEGFP-N1 (Clonetech) or equivalent GFP-expressing plasmid
- Beta-actin (b-actin)-driven luciferase reporter plasmid, or other highly expressing luciferase reporter
Note: Use of a renilla luciferase-expressing plasmid is not recommended in this assay, as we found it interferes with the firefly luciferase signal. - CD3-Alexa700 (eBiosceince, catalog number: 56-0037-42 )
- CD4-PE-Cy7 (BD Biosciences, catalog number: 557852 )
- CD4-PE (Life Technologies, InvitrogenTM, catalog number: MHCD0404 )
- CD45RA–PacificBlue (Life Technologies, InvitrogenTM, catalog number: MHCD45RA28 )
- CD45RO-PerCp-Cy5.5 (BD Biosciences, catalog number: 560607 )
- CD4RA-APC (Life Technologies, InvitrogenTM, catalog number: MHCD45RA05 ) or Alexa647 (BD Biosciences, catalog number: 562763 )
- CD25-APC (Life Technologies, InvitrogenTM, catalog number: MHCD2505 )
- CD40L-FITC (BD Biosciences, catalog number: 555699 )
- CD62L-PE (BD Biosciences, catalog number: 555544 )
- CD69-PE-Cy5 (Life Technologies, InvitrogenTM, catalog number: MHCD6906 )
- MACS buffer (see Recipes)
Equipment
- 96-well round-bottom sterile tissue culture plates (BD Biosciences, catalog number: 353077 or equivalent)
- 96-well white-wall, flat-bottom plates (BD Biosciences, catalog number: 353296 or equivalent)
- MACS LS columns (Miltenyi Biotec, catalog number: 130-042-401 )
- Neon Transfection System (Life Technologies, InvitrogenTM, model: MPK5000 )
- Dynal magnet (Life Technologies, InvitrogenTM, model: 12321D )
- Luminometer with 96-well plate capability (any manufacturer)
- MACS midi magnets (Miltenyi Biotec, model: 130-042-302 )
- Flow cytometer (BD LSR II or similar)
Procedure
Note: All centrifugation steps in 15 or 50 ml conical tubes should be performed at 450 x g for 10 minutes in a general-purpose centrifuge. All centrifugation steps in eppendorf tubes should be performed at 800 x g for 5 min in a microfuge.
- Subject blood to standard Ficoll-Hypaque density gradient centrifugation; harvest interface layer and wash twice in HBSS, and purify with MACS Hunan Naive CD4+ II Kit and LS columns. Collect the flow-through; this is the naive CD4+ T cell fraction. Do not add EDTA to MACS buffer; use only 0.5% BSA in sterile PBS. Count cells. Resuspend in RPMI and centrifuge 540 x g for 10 minutes in general purpose centrifuge. You will need at least 1 x 106 cells for each plasmid to be transfected. To allow for extra volume, stimulate a minimum 1.2 x 107 cells. See Table 1 for a breakdown of cell number requirements.
Table 1. Plasmids and number of replicatesPlasmid Number of Replicates AP-1 luciferase reporter 5 b-actin luciferase reporter 1 pEGFP reporter 1 No plasmid (mock) 3 - Dilute PHA to 2.5 μg/ml in RPMI/10% FBS (no PSG). Concentration should be optimized for each PHA source in terms of transfection efficiency and low expression of activation markers. Resuspend cells in PHA/RPMI at 1 x 106 cells/100 μl. Leave 1 x 106 cells unstimulated for FACS analysis of activation markers.
- Activate cells for 19.5-20 h at 37 °C in a humidified 5% CO2 containing incubator. Set concurrent timers for 19.5 and 20 h. If you are doing more than 10 transfections, stagger the start of the stimulations by at least half an hour to allow enough time to finish transfections by 20 h. The samples must be transfected between 19.5 and 20 h after PHA stimulation.
- Before 19.5 h is up, add DNA plasmids to Eppendorf tubes (2.0 μg/1 x 106 cells), as shown in Table 2. Aliquot 1.0 ml RPMI/10% FBS (no antibiotics) to wells of a 24 well plate (one well for each transfection reaction), and keep at 37 °C until ready to transfect. Add 3.0 ml Neon buffer E (included in Neon kit) to transfection chamber.
Table 2. Volumes for transfectionsTransfection DNA Final volume Total number of reactions buffer T cells necessary 1 3.0 μg 150 μl 1.5 x 106 2 5.0 μg 250 μl 2.5 x 106 3 7.0 μg 350 μl 3.5 x 106 n 2 n + 1 μg 100 n + 50 μl n x 106 + 5 x 105 - At 19.5 h, begin washing cells in PBS by centrifuging for 800 x g for 5 min in microfuge. Resuspend cells in Neon buffer T (106 cells/100 μl, see Table 2). Prepare at least 50 μl extra volume for each tube. Not allowing extra volume will introduce air bubbles into the Neon pipette, which will disrupt the transfection.
- Make sure to finish transfections by 20 h. Set the Neon for 2,400 V, 2 pulses, 12 ms. For other human T cell subsets, these parameters should be optimized as described in the Neon manual.
- Electroporate 100 μl at a time. Check tip to make sure there are no bubbles in the Neon tip, and watch for sparks. If a sample sparks, omit it and do not reuse that tip. Use tips no more than twice. Add each electroporation/transfection reaction (100 μl) to 1 well of 24 well plate. Repeat electroporation until finished. Work quickly to maintain consistency. To assess viability, you may electroporate one sample in the absence of plasmid.
- Incubate cells for 24 h in 37 °C/5% CO2 incubator.
- After 24 or 48 h of activation, stain cells for activation markers. Stain one unstimulated sample and one PHA-stimulated sample. Include aqua amine live/dead discriminator. To stain, pellet 1 x 106 cells in an eppendorf tube (800 x g, 5 min in microfuge), wash with PBS and follow manufacturer's instructions for aqua amine staining. Wash cells in MACS buffer and resuspend in a total volume of 100 μl MACS buffer with antibodies as listed below. Incubate for 10 minutes at room temperature, wash with 1.0 ml of MACS buffer, and resuspend in 1% paraformaldehyde in MACS buffer. Suggested antibodies and fluorophores are listed in the Table 3. This staining combination may be adapted according to the capability of the available flow cytometer.
Table 3. Suggested staining for activation markersEpitope Fluorophore Volume/106 cells CD3 Alexa700 2.5 μl CD4 PE-Cy7 10 μl CD45RA PacificBlue 2.5 μl CD45RO PerCp-Cy5.5 10 μl CD25 APC 2.5 μ CD69 PE-Cy5 2.5 μl CD40L FITC 10 μl CD62L PE 10 μl
Gate on lymphocytes, aqua amine- cells, singlets. To assess purity, gate on CD3+ CD4+ then CD45RA+ CD45RO- cells. To identify the percentage of cells that are not activated in the PHA-stimulated sample, use the unstimulated sample to set gates using a histogram plot for CD25-, CD69-, CD40L-, and CD62L+. See Figure 1 for gating strategy.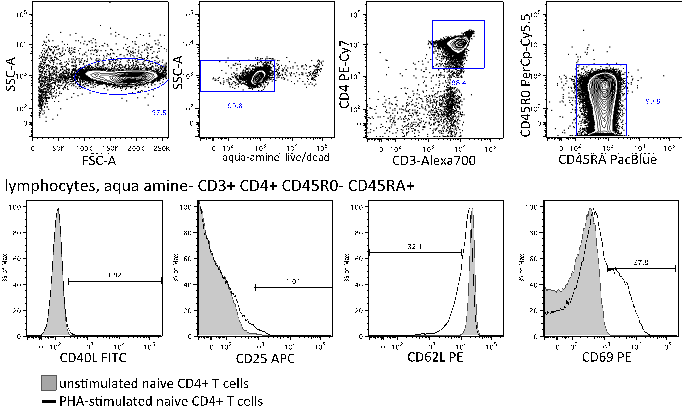
Figure 1. Analysis of sample purity and activation by flow cytometry. Top row: unstimulated sample stained for purity. Bottom row: Overlays of unstimulated (gray) and PHA-stimulated samples (black) stained for activation markers as indicated, and gated on naive CD4+ T cells as indicated. - At 24 h after transfection, activate cells for measurement of AP-1 activity. Wash anti-CD3+anti-CD28-coated Dynal beads with 1 ml RPMI/10% FBS. Leave tube containing beads on magnet for 1 minute before aspirating medium, while leaving tube containing beads on magnet. Remove from magnet, add 1 ml medium, and repeat for a total of 3 washes. Prepare 25 μl beads per 106 cells, plus enough for 1 extra sample. Resuspend in 4x original volume (e.g. 100 μl RPMI/10% FBS per 25 μl beads originally used).
- Dilute and combine PMA and ionomycin to working concentrations of 50 ng/ml PMA and 250 nM ionomycin. Make a total volume of 1 ml.
- Transfer transfected cells to eppendorf tubes and pellet (800 x g, 5 min in microfuge). Leave one replicate of unstimulated cells. Do not combine replicates. Aspirate supernatants and resuspend in 550 μl RPMI/10% FBS. Aliquot 100 μl per well and transfer to 96 well round-bottom plate. Split transfection replicates across rows for 5 wells each replicate. Pellet cells by centrifuging at 450 x g for 10 min and aspirate supernatants.
Note: To avoid this step, you may resuspend cells in 225 μl RPMI/10% FBS and prepare 2x working stocks of all stimulation reagents described above. In this case, aliquot 50 μl of cells per well according to the diagram below and add 50 μl of medium containing stimulation reagents. - Resuspend each well in 100 μl medium containing appropriate stimulation agents and transfer to a 96 well white-wall plate on ice. See Table 4 for layout and diagram of distribution of replicates.
Use RPMI/10% FBS for unstimulated samples, and for b-actin-luciferase-transfected sample. There is room for additional stimuli, such as anti-CD3 alone. If using soluble antibodies, use a secondary cross-linking antibody.
Table 4. Sample plate layout for stimulation1 2 3 4 5 6 7 8 9 10 11 12 A AP-1 (1) medium CD3+CD28 Dynal beads Iono. + PMA B AP-1 (2) medium CD3+CD28 Dynal beads Iono. + PMA C AP-1 (3) medium CD3+CD28 Dynal beads Iono. + PMA D AP-1 (4) medium CD3+CD28 Dynal beads Iono. + PMA E AP-1 (5) medium CD3+CD28 Dynal beads Iono. + PMA F Untransfected medium medium medium medium medium G b-actin-luc medium medium medium medium medium H
- Once all stimulation reagents have been added, remove plate from ice and incubate for 4 h at 37 °C, 5% CO2.
- During stimulation incubation, assess transfection efficiency by staining one untransfected sample and the pEGFP-transfected sample with 2.5 μl of anti-CD4-PE and 2.5 μl of anti-CD4RA-APC or Alexa-647. After staining, add 5 μl 7AAD to each sample to assess viability. This is detected on the PE-Cy5 channel. Do not fix these samples. Transfection efficiency is expected to range between 5 and 40% of live (7AAD-), CD4+ CD45RA+ cells. Set EGFP+ gate at top 1% of untransfected cells. See Figure 2.
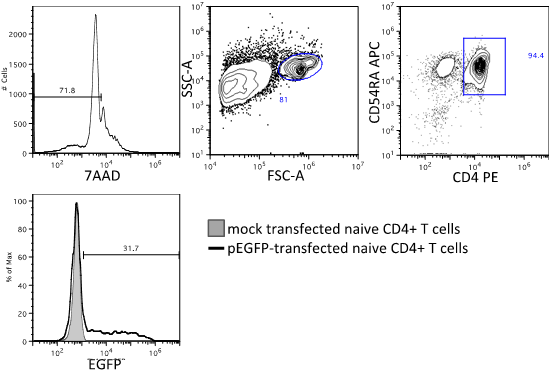
Figure 2. Assessment of viability and transfection efficiency by flow cytometry. Top: mock-transfected sample gated on live cells (7AAD-), then lymphocytes, and CD4+ CD45RA+ cells. Bottom: Measurement of transfection efficiency by %EGFP+ in population described in top row. EGFP+ gate is set at top 1% of signal from mock-transfected cells. - Prepare Promega One-Glo reagent according to the manufacturer's instructions, or thaw an aliquot covered in foil. You will need 100 μl per well. At 4 h after stimulation, remove plate from incubator and add 100 μl One-Glo reagent per well using a multi-channel pipette. Cover with foil and incubate for 3 min.
Note: There is no need to remove Dynal beads as these do not affect the luciferase reading (A. Palin, unpublished data). Read on a luminometer with a 1 sec read per well. One Glo reagent does not require the use of injectors. Include an empty well as a control for background from the plate. - To analyze data, subtract absorbance values for unstimulated samples, pairing the transfection replicates (i.e. subtract A1 value from A2 or A3; B1 from B2 or B3, etc.). Divide instead of subtract to calculate fold change. The untransfected control gives background fluorescence from the cells and the b-actin luciferase signal is a control for luciferase activity. This will be significantly higher than the stimulated cells. The anti-CD3+CD28- stimulated cells give a measure of the upregulation of AP-1 in response to TCR engagement and co-stimulation, while the ionomycin + PMA serves as a measure of the capacity of the cell to induce AP-1. To compare between two sample types or two groups, use the average of the 5 stimulated replicates minus the unstimulated replicates and an unpaired student's t-test.
Recipes
- MACS buffer
1x PBS
0.5% BSA
Sterilize by vacuum filtration.
Note: Do not include EDTA in this buffer, as it may interfere with T cell receptor signaling.
References
- Cron, R. Q., Schubert, L. A., Lewis, D. B. and Hughes, C. C. (1997). Consistent transient transfection of DNA into non-transformed human and murine T-lymphocytes. J Immunol Methods 205(2): 145-150.
- Palin, A. C., Ramachandran, V., Acharya, S. and Lewis, D. B. (2013). Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J Immunol 190(6): 2682-2691.
- Vaysberg, M., Hatton, O., Lambert, S. L., Snow, A. L., Wong, B., Krams, S. M. and Martinez, O. M. (2008). Tumor-derived variants of Epstein-Barr virus latent membrane protein 1 induce sustained Erk activation and c-Fos. J Biol Chem 283(52): 36573-36585.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Palin, A. and Lewis, D. B. (2013). Transfection of Human Naive CD4+ T Cells with PHA Activation and Neon Electroporation. Bio-protocol 3(15): e836. DOI: 10.21769/BioProtoc.836.
Category
Immunology > Immune cell function > Lymphocyte
Molecular Biology > DNA > Transfection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









