- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Maize Endosperm Protein Extraction and Analysis
Published: Vol 3, Iss 14, Jul 20, 2013 DOI: 10.21769/BioProtoc.832 Views: 13847
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Streamlining Protein Fractional Synthesis Rates Using SP3 Beads and Stable Isotope Mass Spectrometry: A Case Study on the Plant Ribosome
Dione Gentry-Torfer [...] Federico Martinez-Seidel
May 5, 2024 2875 Views

An Activity-Based Proteomics with Two-Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE) for Identifying Target Proteases in Arabidopsis Apoplastic Fluid
Sayaka Matsui and Yoshikatsu Matsubayashi
Mar 5, 2025 1995 Views

Advancing 2-DE Techniques: High-Efficiency Protein Extraction From Lupine Roots
Sebastian Burchardt [...] Emilia Wilmowicz
Oct 5, 2025 1776 Views
Abstract
Alcohol-solubility is the most characteristic feature of the zein proteins, the major storage protein in maize. Using sodium borate buffer system with added reducing agent, total proteins are isolated, and zein proteins are separated from non-zein proteins. The extraction effect is intuitive on a SDS-PAGE isolation system. In addition, a simple and rapid approach to extract zeins is introduced, taking full advantage of alcohol-solubility of zeins directly.
Keywords: MaizeMaterials and Reagents
- Mature corn kernels
- Petroleum ether
- Ethanol
- β-mercaptoethanol
- Sodium dodecyl sulfonate (SDS)
- Urea
- Glycerol
- HCl
- Tris(Hydroxymethyl)aminomethane (Tris)
- Phenylmethanesulfonyl fluoride (PMSF)
- Methylene diacrylamide
- Bromophenol blue
- CHAPS (Sigma-Aldrich, catalog number: V900480-5G )
- Dithiothreitol (DTT)
- Liquid nitrogen
- ddH2O
- SDS-PAGE gel (15% separation)
- Coomassie brilliant blue (R250) staining buffer
- 30% acrylamide (see Recipes)
- Sodium borate buffer (see Recipes)
- 5x protein loading buffer (see Recipes)
- IPG solution (see Recipes)
Equipment
- A mortar and pestle
- Centrifuge (Eppendorf, model: 5415D )
- Shaker (Zhicheng, model: ZHWY-111C )
- Concentrator plus (Eppendorf, catalog number: 5305000.193 )
- Gel DocTM XR + System (Bio-Rad, catalog number: 170-8195 )
Procedure
- Using sodium borate buffer system to extract zeins
- Soak 3-5 mature corn kernels in ddH2O for 10 min, then remove the pericarp and embryo and dry the kernels for 10 min at 37 °C.
- Grind kernels into powder using a mortar and pestle within liquid nitrogen.
- Transfer the powder into a 2 ml eppendorf tube. Dry it in Concentrator plus for 1 h till achieve constant weight.
- Add 1 ml petroleum ether. Vortex and place in the shaker at 250 rpm for 1 h.
- Centrifuge for 15 min at 12,000 rpm at room temperature (RT), and discard the supernatant.
- Dry it in Concentrator Plus for 1.5 h until no smell of organic liquid is detectable.
- Fill a new 2 ml Eppendorf tube with 50 mg dried powder from the step 6.
- Add 1 ml sodium borate buffer and 20 μl β-mercaptoethanol as well as 1% PMSF. Mix and incubate with shaking at 250 rpm for at least 2 h at 37 °C.
- Centrifuge for 15 min at 12,000 rpm at RT.
- Transfer 300 μl supernatant into a new 2 ml eppendorf tube as total protein extraction (Fraction A).
- Transfer another 300 μl supernatant from the step 9 into a new 2 ml Eppendorf tube, and add 700 μl ethanol as well as 1% PMSF. Mix with shaking at 250 rpm for 2 h at RT.
- Centrifuge product from step 11 for 15 min at 12,000 rpm at RT.
- Transfer 400-500 μl supernatant into a new 2 ml Eppendorf tube and dry it in Concentrator plus for 2-3 h. Resuspend it in 200 μl IPG as zein proteins extraction (Fraction B).
- Wash the precipitate in step 12 with 70% ethanol twice.
- Centrifuge for 15 min at 12,000 rpm at RT.
- Discard the supernatant and air-dry the precipitate until the edges become transparent. Resuspend it in 200 μl IPG as non-zein proteins extraction (Fraction C).
- Add 10 μl protein extraction from fraction A, or B, or C (see the attached corresponding pictures), 1.5 μl DTT and 3 μl 5x Protein loading buffer in a new 0.2 ml eppendorf tube. Heat 10 min at 99 °C for denaturation.
- Load 2-5 μl denatured protein sample and perform the SDS-PAGE on a 15% separation gel.
- Afterwards, the gel is stained with Coomassie brilliant blue R250 (Figure 1, Figure 2, and Figure 3).
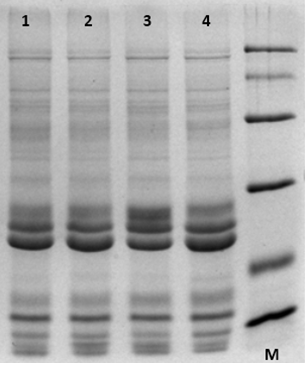
Figure 1. SDS-PAGE (Fraction A). M represents protein standards with molecular weight ranging from 14,400 to 97,400 Da (similarly hereinafter). This figure shows total proteins extraction. 1, 2, 3, 4 represent four different maize cultivars.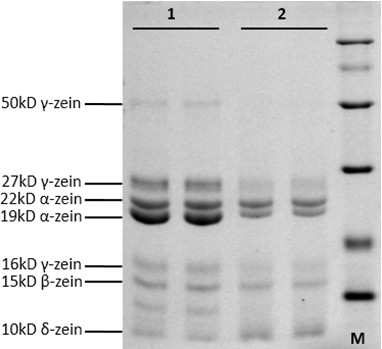
Figure 2. SDS-PAGE (Fraction B). This figure shows zein protein extraction. 1, 2 represent two different maize cultivars (two replicates for each maize cultivar).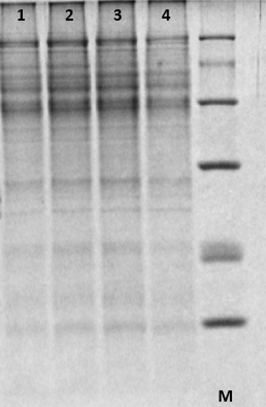
Figure 3. SDS-PAGE (Fraction C). This figure shows non-zein protein extraction. 1, 2, 3, 4 represent four different maize cultivars.
- Soak 3-5 mature corn kernels in ddH2O for 10 min, then remove the pericarp and embryo and dry the kernels for 10 min at 37 °C.
- Simple and rapid extraction approach
- Soak 3-5 mature corn kernels in ddH2O for 10 min, then remove the pericarp and embryo and dry the kernels for 10 min at 37 °C.
- Grind kernels into powder using a mortar and pestle within liquid nitrogen.
- Transfer the powder into a 2 ml eppendorf tube. Dry it in Concentrator plus for 1 h till achieve constant weight.
- Fill a new 2 ml eppendorf tube with 50 mg dry powder from step 3.
- Add 400 μl 70% ethanol and 8 μl β-mercaptoethanol as well as 1% PMSF.
- Mix and incubate for at least 2 h at RT. Invert the cube 2-3 times during incubation.
- Centrifuge for 10 min at 1,300 rpm at RT.
- Transfer 100 μl supernatant into a new 2 ml eppendorf tube.
- Add 10 μl 10% SDS, and mix by pipetting.
- Dry it in Concentrator plus for 1 h.
- Add 200 μl ddH2O for elution.
- SDS-PAGE is performed in 15% polyacrylamide gels, and the gels are stained with Coomassie brilliant blue R250 (Figure 4).
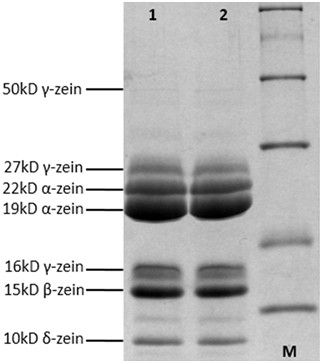
Figure 4. SDS-PAGE (zein proteins). This figure shows zein proteins through simple and rapid extraction approach. 1, 2 represent two replicates of same maize cultivar.
- Soak 3-5 mature corn kernels in ddH2O for 10 min, then remove the pericarp and embryo and dry the kernels for 10 min at 37 °C.
Recipes
- 30% acrylamide (500 ml aqueous solution)
145 g acrylamide
5 g methylene diacrylamide - Sodium borate buffer
12.5 mmol/L sodium borate
1% SDS
pH 10.0 - 5x Protein Loading Buffer
60 mmol/L Tris-HCl (pH 6.8)
25% glycerol
2% SDS
0.1% bromophenol blue - IPG solution
8 mol/L urea
2% CHAPS
References
- Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72: 248-254.
- Gibbon, B. Protein extraction from flour [updated April 2003]. Available from http://ag.arizona.edu/research/larkinslab/protocols.htm
- Wallace, J. C., Lopes, M. A., Paiva, E. and Larkins, B. A. (1990). New Methods for Extraction and Quantitation of Zeins Reveal a High Content of gamma-Zein in Modified opaque-2 Maize. Plant Physiol 92(1): 191-196.
- Wang, G., Sun, X., Wang, G., Wang, F., Gao, Q., Sun, X., Tang, Y., Chang, C., Lai, J., Zhu, L., Xu, Z. and Song, R. (2011). Opaque7 encodes an acyl-activating enzyme-like protein that affects storage protein synthesis in maize endosperm. Genetics 189(4): 1281-1295.
- Wu, Y., Goettel, W. and Messing, J. (2009). Non-Mendelian regulation and allelic variation of methionine-rich delta-zein genes in maize. Theor Appl Genet 119(4): 721-731.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, X., Yao, D. and Song, R. (2013). Maize Endosperm Protein Extraction and Analysis. Bio-protocol 3(14): e832. DOI: 10.21769/BioProtoc.832.
Category
Plant Science > Plant biochemistry > Protein > Isolation and purification
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link