- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Candida albicans Biofilms for Transmission Electron Microscopy
Published: Vol 3, Iss 14, Jul 20, 2013 DOI: 10.21769/BioProtoc.822 Views: 11183
Reviewed by: Fanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Intracellular cAMP Measurements in Candida albicans Biofilms
Liuliu Jiang [...] Xin Wei
Dec 5, 2019 4690 Views

A Novel and Robust Method for Investigating Fungal Biofilm
Biswambhar Biswas [...] Anil Thakur
Jan 5, 2025 1792 Views

Imaging the Entire Sexual Life Cycle of the Budding Yeast Saccharomyces cerevisiae Using a Microfluidic Platform
Taylor Kennedy [...] Orlando Argüello-Miranda
Dec 5, 2025 1480 Views
Abstract
Transmission Electron Microscopy is a form of microscopy that allows for imaging of distinct portions of an individual cell. For Candida albicans biofilms, it is often used to visualize the cell walls of fixed samples of yeast and hyphae. This protocol describes how to grow, harvest, and fix Candida albicans biofilms in preparation for Transmission Electron Microscopy.
Materials and Reagents
- Plate containing grown Candida albicans colonies
- 500 ml 0.22 μm filter (Corning, catalog number: 431118 )
- Aluminum foil
- Bacto-peptone (BD Biosciences, catalog number: 211677 )
- Bacto-yeast extract (BD Biosciences, catalog number: 212750 )
- Cell scraper (BD Biosciences, Falcon®, catalog number: 353085 )
- Difco Dextrose (BD Biosciences, catalog number: 215530 )
- Glutaraldehyde (Sigma-Aldrich, catalog number: G5882 )
- Lead nitrate (PbNO3) (Electron Microscopy Sciences, catalog number: 17900 )
- MOPS (Thermo Fisher Scientific, catalog number: BP308 )
- 4% Osmium tetroxide (Electron Microscopy Sciences, catalog number: 19140 )
- Parafilm
- Paraformaldehyde (Electron Microscopy Sciences, catalog number: 15710 )
- Propylene oxide (Electron Microscopy Sciences, catalog number: 20410 )
- RPMI 1640 (Sigma-Aldrich, catalog number: R6504 )
- Sodium cacodylate trihydrate (Sigma-Aldrich, catalog number: C0250 )
- Sodium chloride (Thermo Fisher Scientific, catalog number: S671-3 )
- Sodium citrate (Electron Microscopy Sciences, catalog number: 21140 , 500 gm)
- Sodium hydroxide (Sigma-Aldrich, catalog number: 59223C )
- Sodium phosphate dibasic anhydrous (Thermo Fisher Scientific, catalog number: BP332-500 )
- Sodium phosphate monobasic monohydrate (Thermo Fisher Scientific, catalog number: S369-500 )
- Spurr’s Resin Kit (Polyscience, catalog number: 01916-1 )
- Uridine (Sigma-Aldrich, catalog number: U3750 )
- Uranyl acetate (Electron Microscopy Sciences, catalog number: 22400 )
- YPD + uridine (see Recipes)
- RPMI + MOPS (see Recipes)
- 1x PBS (see Recipes)
- Fixative (see Recipes)
- Reynold’s Lead Citrate Strain (see Recipes)
Equipment
- 15 ml conical tube (Corning, catalog number: CLS430055 )
- 6 well polystyrene plate (Grenier Bio-one, catalog number: 657160 )
- Hemocytometer
- Shaking incubator with adjustable temperatures and speeds
- Transmission Electron Microscope facility with trained microscopist
Procedure
- 5 ml of YPD + uridine culture in a 15 ml conical tube is inoculated with a single colony of Candida albicans growing on a plate that is between 2 and 10 days old (age of colony may vary based on growth rate of particular strain). Most Candida strains and species should grow well in this medium, but if another medium is commonly used for a specific strain, that can be used instead.
- Inoculum is incubated overnight at 30 °C and 200 rpm.
- Cell concentration of overnight culture is determined by cell counting on a hemocytometer.
- Inoculum diluted to 1 x 106 cells/ml in RPMI + MOPS.
- 1 ml of diluted incolulum added to each well of a 6 well plate.
Note: Inoculum spread to cover the entire bottom of the well. - 6 well plate incubated at 30 °C for 60 min without shaking.
- Inoculum is then removed from each well gently, so as not to scratch away the newly forming biofilm.
- 1 ml of fresh RPMI + MOPS media is added gently to each well.
- 6 well plates are wrapped in parafilm and aluminum foil and incubated for 24 h at 37 °C and 50 rpm.
Note: It is very important that the biofilms not be shaken above 50 rpm, as this will dislodge the growing biofilm from the plate bottom. - After 24 h, media is gently removed so as not to disturb the biofilm and replaced with 1 ml of fresh RPMI + MOPS.
Note: Tilting the 6 well plates and gently placing your pipet tip in the corner of the well helps to prevent too much disruption of the fragile biofilm. - 6 well plates are re-wrapped as before and incubated another 24 h at 37 °C and 50 rpm.
- After the second 24 h incubation, the media is removed from the wells and the biofilms are gently washed 1x in 1 ml of 1x PBS. It is important to slowly pipet in the PBS so as not to disturb the delicate biofilms. The PBS wash can be removed immediately after adding it to the biofilm.
- Harvest each biofilm in 2 ml of 1x PBS and store in a 15 ml conical tube.
Note: Harvesting is best done by adding 1 ml of PBS into the well and scraping away the biofilm with a plastic spatula, and adding the biofilm to the conical tube. Then repeat this process with the second 1 ml of PBS to collect any remnants.
- Conical tubes are gently centrifuged at 720 x g for 5 min at room temperature.
- Supernatant is discarded and the pellet is resuspended in 2 ml of Fixative.
- Tubes can be stored at 4 °C until ready for imaging by a trained transmission electron microscopist. It is recommended that the tubes not be stored more than a few days.
- The following steps are a summary of those performed by a trained transmission electron microscopist.
- Cells are postfixed in 1% osmium tetroxide.
- Samples are then dehydrated by soaking for 10 min each in increasing concentrations of ethanol (30%, 50%, 70%, 85%, 90%, 95%, and 100%).
- Next, samples are rinsed 3 x 10 min in 100% propylene oxide then embedded in Spurr’s resin.
- 70 nm sections are cut and placed on copper grids and poststained for 5 min with 8% uranyl acetate in 50% ethanol and for 5 min in Reynold’s lead citrate stain.
- Samples can now be analyzed by TEM. A sample picture of a reference strain Candida albicans biofilm is shown below (Figure 1). Most pictures will only show one or two cells at a time, and usually cross-sectioned so that you can see inside the cells. Scale bar represents 1 μm.
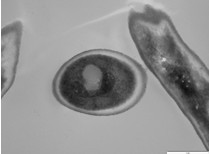
Figure 1. Transmission Electron Microscopy image of a Candida albicans biofilm cell
- Cells are postfixed in 1% osmium tetroxide.
Recipes
- YPD + uridineCombine all ingredients and aliquot into 100 ml bottles. Autoclave bottles on liquid cycle to sterilize.
Bacto-yeast extract (1%) 10 g Bacto-peptone (2%) 20 g Dextrose (2%) 20 g Uridine 0.08 g Distilled water 1,000 ml - RPMI + MOPSCombine all ingredients in a 2 L flask. Use a 10 ml pipette to adjust pH to 7.0 using Sodium hydroxide (5 N). Bring volume up to 1 L with distilled water. Filter sterilize RPMI + MOPS into 500 ml bottles. Use at 37 °C, but can store for up to 6 months at 4 °C to prevent contaminant growth.
RPMI 1640 10.4 g MOPS 34.5 g Distilled water 700 ml - 1x PBS
Add above ingredients to 450 ml distilled water. Adjust pH to 7.4 by adding 1 M NaOH if necessary. Bring final volume to 500 ml distilled water. This makes 10x PBS stock. Dilute in distilled water 1:10 for final concentration of PBS.Sodium phosphate monobasic monohydrate (mw = 137.99; 0.038 M) 2.62 g Sodium phosphate dibasic anhydrous 11.5 g Sodium chloride 43.84 g Distilled water 500 ml - Fixative
2.5% glutaraldehyde
2.0% paraformaldehyde
Add above ingredients to 0.1 M sodium cacodylate trihydrate. Use caution with this reagent, as it is very toxic. - Reynold’s lead citrate stainPlace all ingredients in a 50 ml flask and shake forcefully for 1 min, then intermittently for 30 min until fully dissolved. Then add 8 ml of NaOH and bring volume up to 50 ml with distilled water. Filter sterilize before using.
Lead nitrate 1.33 g Sodium citrate 1.76 g Distilled water 30 ml 1 N Sodium hydroxide 8 ml
References
- Bowling Green State University (2012). Reynold’s Lead Citrate Stain. Retrieved from www.bgsu.edu/departments/biology/facilities/MnM/TEM/ReynoldsLeadStain.pdf.
- Nett, J. E., Sanchez, H., Cain, M. T., Ross, K. M. and Andes, D. R. (2011). Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell 10(12): 1660-1669.
- Nett, J., Lincoln, L., Marchillo, K., Massey, R., Holoyda, K., Hoff, B., VanHandel, M. and Andes, D. (2007). Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51(2): 510-520.
- Taff, H. T., Nett, J. E., Zarnowski, R., Ross, K. M., Sanchez, H., Cain, M. T., Hamaker, J., Mitchell, A. P. and Andes, D. R. (2012). A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog 8(8): e1002848.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Taff, H. T. and Andes, D. R. (2013). Preparation of Candida albicans Biofilms for Transmission Electron Microscopy. Bio-protocol 3(14): e822. DOI: 10.21769/BioProtoc.822.
Category
Microbiology > Microbial biofilm > Biofilm culture
Microbiology > Microbial cell biology > Cell imaging
Cell Biology > Cell imaging > Electron microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link