- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Endosomal pH Measurement in Bone Marrow Derived Dendritic Cells
Published: Vol 3, Iss 14, Jul 20, 2013 DOI: 10.21769/BioProtoc.819 Views: 10182
Reviewed by: Fanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Salivary Charged Metabolites Using Capillary Electrophoresis Time-of-flight-mass Spectrometry
Masahiro Sugimoto [...] Tomoyoshi Soga
Oct 20, 2020 4630 Views

Detection and Quantification of Calcium Ions in the Endoplasmic Reticulum and Cytoplasm of Cultured Cells Using Fluorescent Reporter Proteins and ImageJ Software
Shunsuke Saito and Kazutoshi Mori
Aug 20, 2023 2854 Views

In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay
Sudhakar Singh [...] Sharvan Sehrawat
Apr 20, 2025 4366 Views
Abstract
Endosomes embraces different set of compartments such as early endosomes, intermediate endosomes and late endosomes or lysosomes. They become acidic as they mature. This acidification is generated by the vacuolar membrane proton pump V-ATPase that is recruited in late endosomes. This protocol described the measurement of endosomal pH using dextran molecules labelled with pH sensitive and insensitive dyes.
Materials and Reagents
- CO2 independent medium (Invitrogen, catalog number: 18045054 )
- Iscove’s Modified Dulbecco’s Medium (IMDM) (Sigma-Aldrich, catalog number: I3390 )
- 10% fetal bovine serum (FBS) (Hyclone/PAA, catalog number: sv143-03 )
- Penicillin-streptomycin (100 Units/ml, 100 μg/ml) (Sigma-Aldrich, catalog number: P11-010 )
- Glutamine (Sigma-Aldrich, catalog number: G75013 )
- 2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Granulocyte-macrophage colony stimulating factor (GM-CSF) (Peprotech, catalog number: 315-03 )
- 40,000 MW Dextran fluorescein (10 mg/ml) (Molecular Probes)
- 40,000 MW Dextran Alexa 647 (10 mg/ml) (Molecular Probes)
- 5 mM EDTA (Invitrogen, catalog number: 15575-038 )
- 1% Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Triton X-100 (Sigma-Aldrich, X-100) kept at room temperature
- 1x PBS (see Recipes)
- Conditioned complete medium (see Recipes)
Equipment
- Water bath (37 °C)
- Incubator (37 °C)
- The FACSCalibur flow cytometer (Becton Dickinson)
- Hemocytometer
- Centrifuge
Procedure
- Detach bone marrow-derived dendritic cells (BMDCs) with 1x PBS-5 mM EDTA (10 min at 37 °C).
- Wash the cells with 1x PBS twice by centrifugation at 367 x g for 10 min.
- Count cells. A total of 3 x 106 cells are required for pH measurement at different time points (kinetics of 10 min, 20 min, 30 min and 60 min for example). Additionally, 6 x 106 cells are needed to acquire the pH standard curve.
- For measurement of pH at different time points, resuspend the cells in 100 μl total volume of prewarmed conditioned complete medium containing 1 mg/ml of fluorescein- and 0.5 mg/ml Alexa-647-labeled 40,000 MW dextrans. Pulse cells in a water bath set at 37 °C for 10 min.
- Stop the reaction by adding a large volume (1 ml) of cold 1x PBS-1% BSA and wash cells extensively (6 times) with the same buffer to get rid of the non-internalised dextrans.
- After washing, resuspend the cells in prewarmed conditioned complete medium (1 ml) and incubate at 37 °C for different time points (chase). The pulse (10 min) corresponds to early endosomes (EE), the chase of 40 min corresponds to intermediate endosomes (IE) and the 110 min of chase corresponds to the lysosomes.
- Stop the reaction each time by immediately adding cold PBS and placing the tubes on ice.
- Rapidly, analyse the cells by FACS, via a FL1/FL4 gate selective for cells that have endocytosed both fluorescent probes and determine the ratio of the mean fluorescence intensity (MFI) emission between the two probes.
- For the pH standard curve, resuspend the cells in 200 μl total volume of prewarmed conditioned complete medium containing 1 mg/ml of fluorescein- and 0.5 mg/ml Alexa-647-labeled 40,000 MW dextrans and pulse the cells in a water bath set at 37 °C for 20 min. Repeat step 5 and split cells into nine 1.5 ml Eppendorf tubes for a pH measurement ranging from 4 to 8.
- Prepare several buffers that differ in pH by 0.5 units using prewarmed CO2 independent medium. Adjust the pH with citric acid or NaOH.
- Resuspend each cell pellet in a different pH solution supplemented with 0.001% of Triton X-100 to slightly permeabilise the cells and to give access to the external prefixed pH solution into the cell.
- Analyse immediately by FACS and determine the ratio of the mean fluorescence intensity (MFI) emission between the two fluorescent probes at each pH. Make the standard curve by plotting the different MFI ratio values that correspond to each pH and apply this formula to the MFI ratio values obtained before (steps 1-8, Figure 1).
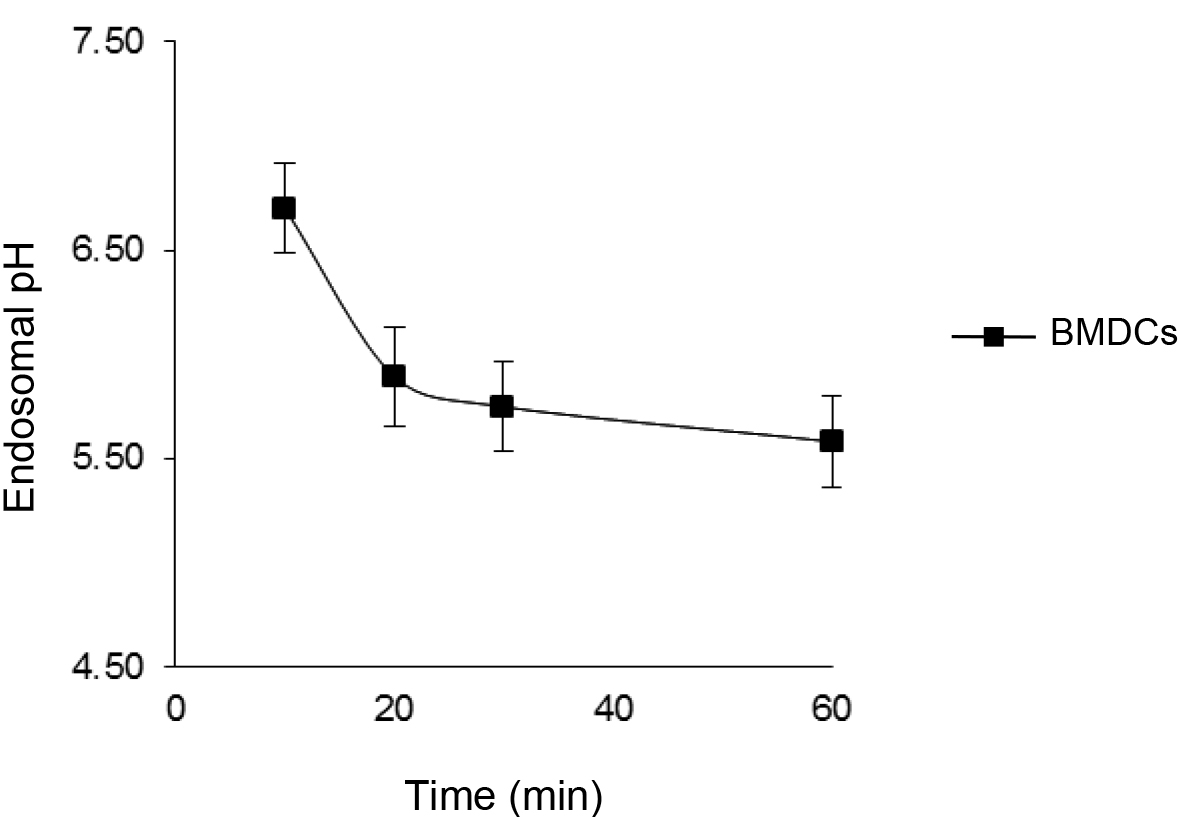
Figure 1. Kinetic of endo-lysosomal pH in BMDCs pulsed with a mixed of fluorescein- and Alexa-647-labeled 40,000 MW dextrans for 10 min and then chased for different times
Recipes
- 1x PBS
137 mM NaCl
2.7 mM KCl
8 mM Na2HPO4
1.46 mM KH2PO4
Keep 1x PBS cold - Conditioned complete medium
IMDM supplemented with
10% FBS
100 Units/ml,100 μg/ml penicillin-streptomycin
2 mM glutamine
50 μM 2-mercaptoethanol
10 ng/ml GM-CSF
Acknowledgments
This protocol is adapted from Savina et al. (2010); Maschalidi et al. (2012) and Sepulveda et al. (2009).
References
- Maschalidi, S., Hassler, S., Blanc, F., Sepulveda, F. E., Tohme, M., Chignard, M., van Endert, P., Si-Tahar, M., Descamps, D. and Manoury, B. (2012). Asparagine endopeptidase controls anti-influenza virus immune responses through TLR7 activation. PLoS Pathog 8(8): e1002841.
- Savina, A., Vargas, P., Guermonprez, P., Lennon, A. M. and Amigorena, S. (2010). Measuring pH, ROS production, maturation, and degradation in dendritic cell phagosomes using cytofluorometry-based assays. Methods Mol Biol 595: 383-402.
- Sepulveda, F. E., Maschalidi, S., Colisson, R., Heslop, L., Ghirelli, C., Sakka, E., Lennon-Dumenil, A. M., Amigorena, S., Cabanie, L. and Manoury, B. (2009). Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity 31(5): 737-748.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Maschalidi, S. and Manoury, B. (2013). Endosomal pH Measurement in Bone Marrow Derived Dendritic Cells. Bio-protocol 3(14): e819. DOI: 10.21769/BioProtoc.819.
- Maschalidi, S., Hassler, S., Blanc, F., Sepulveda, F. E., Tohme, M., Chignard, M., van Endert, P., Si-Tahar, M., Descamps, D. and Manoury, B. (2012). Asparagine endopeptidase controls anti-influenza virus immune responses through TLR7 activation. PLoS Pathog 8(8): e1002841.
Category
Immunology > Immune cell function > Dendritic cell
Biochemistry > Other compound > Ion
Cell Biology > Cell-based analysis > Ion analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link