- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation, Culture and Differentiation of Primary Acinar Epithelial Explants from Adult Murine Pancreas
Published: Vol 3, Iss 13, Jul 5, 2013 DOI: 10.21769/BioProtoc.818 Views: 12404
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1255 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 231 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 144 Views
Abstract
The adult pancreas possesses an intrinsic developmental plasticity whereby acinar cells can convert into ductal structures under some pathological conditions. Acinar tissue can be isolated from murine pancreas and kept in three-dimensional collagen culture. Acinar to ductal metaplasia can be induced in primary acinar epithelial explants by treatment with growth factors. This method can be utilized in ex vivo studies involving pancreatic epithelial differentiation.
Keywords: Pancreatic aciniMaterials and Reagents
- Mice (4-7 weeks old). As a positive control you can always use wild type mice and induce differentiation of acinar explants by treatment with EGF as described. Depending on your application you may use transgenic mice.
- McCoy′s 5A medium (Sigma-Aldrich, catalog number: M8403 )
- Waymouth′medium MB 752/1 medium (Genaxxon, catalog number: C4119 )
- BSA (Sigma-Aldrich, catalog number: A4503 )
- Soybean trypsin inhibitor (SBTI) (Sigma-Aldrich, catalog number: T6522 )
- Collagenase Type VIII (Sigma-Aldrich, catalog number: C2139 )
- 80% EtOH
- Penicillin-streptomycin (P/S) (Life Technologies, Gibco®, catalog number: 15140-122 )
- Amphotericin B (not critical, for example from Biochrom, catalog number: A2610 )
- FCS (Life Technologies, Gibco®, catalog number: 10270 )
- Bovine pituary extract (BPE) (Life Technologies, Gibco®, catalog number: 13028-014 )
- Insulin, Transferrin, Selenium, Ethanolamine solution (ITS-X) (Life Technologies, Gibco®, catalog number: 51500 )
- Rat Tail Collagen Type I (BD Biosciences, catalog number: 354236 )
- Sterile 10x PBS (BIOCHROM, catalog number: 182-50 )
- Sterile dH2O
- Sterile 1 N NaOH
- rhTGFa (R&D systems, catalog number: 239-A )
- mEGF (BD Biosciences, catalog number: 354010 )
- SBTI stock solution (see Recipes)
- Collagenase VIII stock solution (see Recipes)
- Wash solution (see Recipes)
- 0.1% Culture medium (Waymouth′s medium) (Genaxxon, catalog number: C4119) (see Recipes)
- 30% Culture medium (Waymouth′s medium) (Genaxxon, catalog number: C4119) (see Recipes)
Equipment
- Stericups filter units (EMD Millipore)
- 50 ml Falcon tubes
- Tissue culture dishes (10 mm)
- 6-well plates
- 24- or 48-well plates
- Dissecting forceps and scissors
- Disposable scalpels
- 100 μM cell strainer (BD Biosciences, Falcon®, catalog number: 352360 )
- Syringe plungers
Procedure
I. On the bench:
Mice should be between 4 and 7 weeks of age. Isolation of acinar issue does not work with older mice. Because time is a critical step for the viability of acinar cells, we usually do not process more than 2 mice at the same time.
From one mouse it is possible to obtain about 1,000 acinar-explants. Acinar tissue can be seeded for example in 15 wells of a 48-well plate, thus the obtimal denisty for differetiation of acinar tissue is obtained when you see about 30 acinar explants in a well through a 10x objetive. If density is too high differentiation will take longer, if too low acinar cells will die.
- Put forceps and scissors into 80% EtOH.
- Anesthetize the mouse with isofluorane, sacrifice the mouse by cervical dislocation and resect the pancreas as fast and possible (time is a critical step).
- Place the resected pancreas into a tissue culture dish containing ice cold sterile PBS.
II. Up to now all protocol steps should be performed under sterile conditions.
Centrifugation steps at 720 x g (18 °C) are a critical step!
- In the hood, transfer the pancreas into a culture dish containing 10 ml of PBS.
- Immediately put the pancreas in culture dish with 5 ml collagenase VIII solution (see Recipe 4) and cut the organ in very small pieces (less then 1 mm of diameter) with a scalpel within 2 min. Pipette the tissue up and down.
- Incubate the dish at 37 °C for 10 min. Shake from time to time.
- Transfer the sample into a 50 ml falcon. Pipette the solution 3x up and down.
- Wash the dish with 10 ml wash solution and transfer to the 50 ml falcon.
- Centrifuge, 720 x g, 5 min, 18 °C.
- Carefully remove the supernatant.
- Resuspend the pellet in 5 ml collagenase VIII solution. Put in a culture dish. Pipette the tissue up and down.
- Incubate at 37 °C for 10 min. Shake from time to time.
- Aspirate the sample and filter through a 100 μm nylon cell strainer positioned on a 50 ml falcon.
- Macerate the tissue pieces through the cell strainer with a syringe plunger.
Note: This step is difficult to describe, try to press the tissue through the strainer with the syringe plunger without applying a shear stress. It is possible you have to optimize this step in your hands.
- Wash the mesh with 10 ml wash solution to carefully collect any remaining cell.
- Centrifuge, 720 x g, 5 min, 18 °C.
- Carefully remove the supernatant.
- Aspirate the pellet in 20 ml wash solution, and transfer the solution in a fresh tube. Do not aspirate up and down!
- Centrifuge, 720 x g, 5 min, 18 °C.
- Resuspend the pellet in 2 ml culture medium 30% and transfer it on a well of a 6-well culture dish.
- Check the quality of isolated acinar tissue under the microscope. Isolation is good if you see clusters of acinar cells (epithelial explants, the morphology is the same as in the Ctr. of the figure below) and only rare isolated acinar cells swimming around. Sometimes damaged acinar cells agglomerate together, remove such sticky agglomerates with a forceps.
- Let the isolated acini recover for 60 min in the incubator (37 °C with 5% CO2).
- Meanwhile precoat a cell culture plate of the desired size with collagen (see below). Calculate the required volume of collagen - medium mixture required for step 25. The volume depends from your application. Calculated 100 μl volume/well for a 48-well plate or 80 μl volume/well for an 8 well chamber slide. Typically you will seed the acinar explants obtained from one wild type mice in 15 wells of a 48 well plate. In this case you need 100 μl x 15/2 = 750 μl collagen and 100 μl x 15/2 = 750 μl medium 0.1%.
- Collect the acinar suspension from the plate and transfer it to a fresh 15 ml tube.
- Centrifuge acinar suspension, 720 x g, 5 min, 18 °C.
- Carefully aspirate the supernatant.
- Resuspend the pellet in a mixture of collagen and culture medium 0.1% (1:1 vol. /vol.)
- Pipet the acini/collagen-medium suspension mixture into each coated well in the plate (at least in triplicate). For the typical 48-well example you pipet 100 μl of the mixture in 15 wells.
- Wait until solidification of the collagen (about 30 min)
- Add some more collagen (100 μl/well for the 48 well plate). You may skip this step when you will perform immunofluorescence as a downstream application. Wait until solidification (about 30 min)
- Add culture medium 0.1% with/without the desired supplements to each well. Typically 400 μl/well for a 48 well plate.
- To induce transdifferentiation of acinar explants, add TGFa (10 ng ml-1) or EGF (25 ng ml-1) into the culture medium 0.1% at day 1-3-5 where day 1 is the day of isolation.
Note:
1) We usually quantify transdifferentiation of wild type acinar explants after EGF treatment at day 5 (Figure 1).
2) Culture can be kept until day 8. For longer periods of culture you have to reseed the acinar explants into fresh collagen because cultures become acidic and collagen breaks down.
III. Gelation Procedure for Rat Tail Collagen I, 2.5 mg ml-1
- Place on ice: Collagen, sterile 10x PBS, sterile water, sterile 1 N NaOH, falcon tubes. Keep all reagents on ice.
- Calculate the total amount of collagen (final concentration of 2.5 mg ml-1) needed with the formula: (final Vol. x 2.5)/ (collagen concentration in the bottle). You need a 100/120 μl collagen layer/well for a 48-well plate or 80 μl collagen layer/well for a 8 well chamber slide.
- Prepare a tube on ice with the following volume of 10x PBS: (final Vol.)/10.
- Add the following volume of 1 N NaOH to the tube containing 10x PBS: (Vol. collagen to be added) x 0.023 ml.
- Add to the 10x PBS/1 N NaOH the following volume of sterile ice-cold water: (final Vol.) - (Vol. collagen) - (Vol. 10x PBS) - (Vol. 1 N NaOH).
- Mix the contents of tube and hold in ice.
- Add the calculated volume of collagen and mix. Leave on ice (stable for 2-3 h) until ready for use.
- Coat tissue culture dishes with collagen (use 24/48 wells for quantification of transdifferentiation, protein or RNA extraction, or use 8 well chamber slides for later staining applications).
- Allow collagen to solidify at 37 °C (about 30 min)
Note: Downstream applications include immunofluorescence, protein extraction, RNA extraction and cytotoxicity assay.
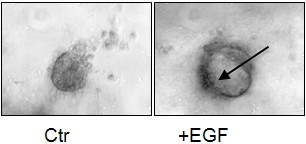
Figure 1. Example of acinar explants at day 5. Acinar explants from a wild type mouse were isolated and cultivated for 5 days in collagen as described. Ctr (medium only): a typical acinar explant is composed by a sphere of several acinar cells. EGF: in presence of 25 ng/ml of EGF cell clusters get a more flattened morphology and differentiate to a duct-like structure characterized by a lumen (arrow) lined by several cells.
Recipes
- SBTI stock solution
10 mg/ml in McCoy medium, store at -20 °C.
- (optional) Collagenase VIII stock solution
9.6 mg/ml in McCoy medium (not fully dissolved), store at -20 °C.
- Wash solution (40 ml/mouse)
McCoy′s medium
0.1% BSA (sterile filtered)
0.2 mg/ml SBTI (Stock 1:50)
- Collagenase VIII solution (10 ml/mouse)
McCoy′s medium
0.1% BSA (sterile filtered)
0.2 mg/ml SBTI (Stock 1:50)
1.2 mg/ml Collagenase VIII (critical step, add collagenase just before use, dilute the collagenase VIII stock solution 1:8)
- 0.1% culture medium
Waymouth′s medium
0.1% BSA (sterile filtered)
0.2 mg/ml SBTI (Stock 1:50)
P/S 1:200 (optional, not necessary for short term culture)
0.25 μg/ml Amphotericin B (antifungal, optional)
ITS-X 1:100
BPE: 50 μg/ml (dilute according the batch concentration) (Aliquots, -20 °C)
0.1% FCS
- 30% culture medium
0.1% culture medium
30% FCS
Acknowledgments
This protocol for acinar epithelial explants from adult murine pancreas was established by modification previously published protocols ((Lisle and Logsdon, 1990; Githens et al., 1994; Means et al., 2005). The work was supported by grants from the Wilhelm Sander Stiftung (2010.021.1).
References
- Ardito, C. M., Grüner, B. M., Takeuchi, K. K., Lubeseder-Martellato, C., Teichmann, N., Mazur, P. K., DelGiorno, K. E., Carpenter, E. S., Halbrook, C. J. and Hall, J. C. (2012). EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22(3): 304-317.
- Githens, S., Schexnayder, J. A., Moses, R. L., Denning, G. M., Smith, J. J. and Frazier, M. L. (1994). Mouse pancreatic acinar/ductular tissue gives rise to epithelial cultures that are morphologically, biochemically, and functionally indistinguishable from interlobular duct cell cultures. In Vitro Cell Dev Biol Anim 30A(9): 622-635.
- Heid, I., Lubeseder–Martellato, C., Sipos, B., Mazur, P. K., Lesina, M., Schmid, R. M. and Siveke, J. T. (2011). Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology 141(2): 719-730. e717.
- Lisle, D., Ratcliffe, J. F., Faoagali, J. and Cherian, S. (1990). Bacterial contamination of contrast media stored after opening. Br J Radiol 63(751): 532-534.
- Means, A. L., Meszoely, I. M., Suzuki, K., Miyamoto, Y., Rustgi, A. K., Coffey, R. J., Wright, C. V., Stoffers, D. A. and Leach, S. D. (2005). Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132(16): 3767-3776.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lubeseder-Martellato, C. (2013). Isolation, Culture and Differentiation of Primary Acinar Epithelial Explants from Adult Murine Pancreas. Bio-protocol 3(13): e818. DOI: 10.21769/BioProtoc.818.
Category
Cell Biology > Tissue analysis > Tissue isolation
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










