- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Malondialdehyde, Chlorophyll Proline, Soluble Sugar, and Glutathione Content in Arabidopsis seedling
Published: Vol 3, Iss 14, Jul 20, 2013 DOI: 10.21769/BioProtoc.817 Views: 34934
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3931 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2052 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
The protocol has four sub-protocols, which are about the measurement of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content, respectively, in Arabidopsis seedling by using spectrophotometer. These methods are simple, effective and reproducible, which will help the researchers who are not familiar with these approaches, quickly get reliable results.
Keywords: MalondialdehydeI. Measurement of Malondialdehyde
Materials and Reagents
- Thiobarbituric acid (TBA) (Sigma-Aldrich, catalog number: T5500 )
- Trichloroacetic acid (TCA) (Sigma-Aldrich, catalog number: T9159 )
- Malondialdehyde (MDA) (BOC Sciences, catalog number: 542-78-9 )
Equipment
- Centrifuge
- Spectrophotometer
Procedure
Note: The experiment is done at room temperature (RT) except of specific indication.
- 0.1 g leaf tissue (with similar age, and young expanded leaf may be better) is ground into powder with liquid nitrogen, and then put the powder into a tube containing 1 ml 0.1% (w/v) TCA and mix by inverting the tube to homogenize the leaf tissue.
- Centrifuge homogenized samples at 10,000 x g for 10 min, and then transfer supernatant to a new tube.
- 4 ml of 20% TCA containing 0.5% TBA was added to the supernatant and mixed well.
- The mixture is boiled at 95 °C for 15 min and quickly cooled on ice (TBA can interact with MDA and results into red compound in acidic buffer, so the content of MDA can be calculated by measuring the density of the resulting red compound with spectrophotometer at 532 nm. The high temperature can accelerate the reaction and low temperature can inhibit it).
- Centrifuge the mixture at 10,000 x g for 5 min, and then transfer supernatant to a new tube.
- To generate a standard curve, a serial concentration of MDA is made: 1 μM, 2 μM, 5 μM, 10 μM, 20 μM and 50 μM (the volume of each dilution depends on the size of the cuvette of spectrophotometer).
- Measure the optical density of standard samples from step 6 at 532 nm by spectrophotometer and make the standard curve to get the extinction coefficient (Figure 1).
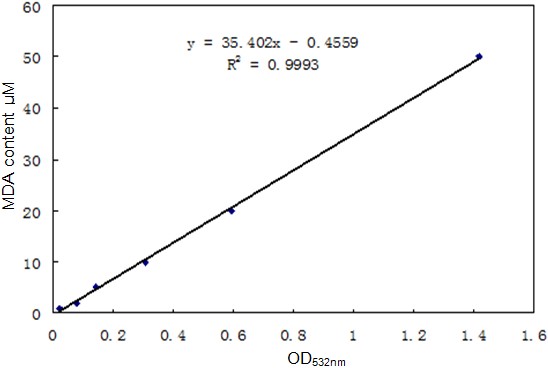
Figure 1. The standard curve of MDA - Measure the optical density of plant samples from step 5 at 532 nm and calculate the content of MDA according to the standard curve (Madhava Rao and Sresty, 2000; Baryla et al., 2000).
II. Measurement of chlorophyll
Materials and Reagents
- Dimethyl formamide (DMF) (Sigma-Aldrich, catalog number: D4551 )
Equipment
- Centrifuge
- Spectrophotometer
Procedure
Note: The experiment is done at room temperature.
- 0.1 g leaf tissue is ground into powder with liquid nitrogen, and then homogenized with 1 ml 100% DMF.
- Centrifuge homogenized samples at 10,000 x g for 10 min, and then gather the supernatant.
- Measure the optical density of the supernatant at 664 nm and 647 nm, respectively.
- Calculate the content of chlorophyll a and chlorophyll b by the following formulas (Sibley et al.,1996; Inskeep and Bloom, 1985; Aono et al., 1993):
[chlorophyll a] = 12.7 x A664 - 2.79 x A647
[chlorophyll b] = 20.7 x A647 - 4.62 x A664
[chlorophyll a + chlorophyll b] = 17.90 x A647 + 8.08 x A664
III. Measurement of Proline
Materials and Reagents
- Sulphosalicylic acid (DingGuo, catalog number: DS094 )
- Proline (Sigma-Aldrich, catalog number: 858919 )
- Ninhydrin (Sigma-Aldrich, catalog number: 151173 )
- Acetic acid (DingGuo, catalog number: DS002 )
- Orthophosphate (Sigma-Aldrich, catalog number: P2023 )
- Toluene (Sigma-Aldrich, catalog number: 650579 )
- Ninhydrin reagent (see Recipes)
Equipment
- Centrifuge
- Spectrophotometer
Procedure
Note: The experiment is done at room temperature (RT) except of specific indication.
- To generate a standard curve, a serial concentration of Proline is made in 3% sulphosalicylic acid: 1 μM, 10 μM, 50 μM, 100 μM, 150 μM, 200 μM, 300 μM, 1 ml for each dilution.
- Each 500 μl standard solution is added with 500 μl acetic acid and 500 μl ninhydrin reagent in 5 ml tube and boil for 45 min, and then cooled in ice for 30 min.
- Add equal volume toluene to each sample and vibrate for 1 min, and then centrifuge at 1,000 x g for 5 min.
- Measure the optical density of toluene solution at 520 nm by spectrophotometer and make the standard curve (Figure 2).
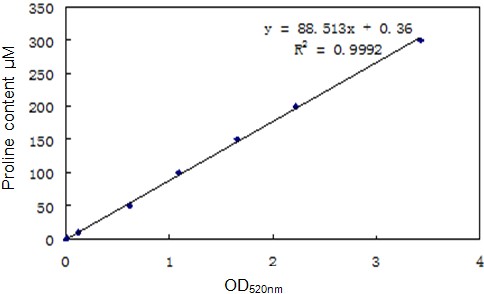
Figure 2. The standard curve of proline
- 0.5 g plant sample is ground into powder with liquid nitrogen, and then homogenized with 2 ml of 3% sulphosalicylic acid in tube.
- Centrifuge homogenized samples at 5,000 x g for 5 min, and then collect the supernatant
- The supernatant is treated as steps 2 and 3, and measure the optical density of samples as step 4, and then calculate the content of praline using the standard curve from step 3 (Bates et al., 1973; Lattanzioa et al., 2009).
Recipes
- Ninhydrin reagent
2.5 g ninhydrin is successively added to 60 ml Glacial Acetic acid and 40 ml 6 M orthophosphate, and then dissolved at 70 °C. After cool down, the reagent can be stored in brown bottle at 4 °C for less than 24 h.
IV. Measurement of Soluble Sugar
Materials and Reagents
- Ethanol (DingGuo, catalog number: DS023 )
- Glucose (DingGuo, catalog number: DS063 )
- Anthrone (SCRC, catalog number: 30015014 )
- H2SO4 (Sigma-Aldrich, catalog number: 339741 )
- Thiourea (Amresco, catalog number: M222 )
- Chloroform (Sigma-Aldrich, catalog number: C2432 )
- Anthrone reagent (see Recipes)
Equipment
- Centrifuge
- Spectrophotometer
- Shaker
Procedure
Note: The experiment is done at room temperature (RT) except of specific indication.
- To generate a standard curve, a serial concentration of glucose is made: 1 μM, 10 μM, 50 μM, 100 μM, 150 μM, 200 μM, 300 μM, 5 ml for each concentration of glucose solution.
- 50 μl of each diluted glucose solution is mixed with 4.95 ml anthrone reagent and then boiled for 15 min.
- Measure the optical density of glucose standards at 620 nm by spectrophotometer to generate a standard curve.
- 0.1 g dried sample is ground into powder with liquid nitrogen, and then homogenized with 2 ml 80% ethanol in shaker at 200 rpm in 50 ml tube for 1 h.
- Centrifuge at 6,000 x g for 10 min, and then transfer as much supernatant as possible into a new 5 ml tube.
- Add equal volume of chloroform, completely mix, and then centrifuge at 12,000 x g for 10 min.
- The aqueous part is transferred to a new tube and repeat steps 2 and 3 to measure the optical density of the sample. The content of soluble sugar is calculated according the standard curve made at step 3 (Mandre et al., 2002; Jin et al., 2007).
Recipes
- Anthrone reagent
72% H2SO4
500 mg/L anthrone
10 g/L thiourea
V. Measurement of Glutathione
Materials and Reagents
- Trichloroacetic acid (TCA) (Sigma-Aldrich, catalog number: T9159 )
- Polyvinylpolypyrrolidone (PVPP) (Sigma-Aldrich, catalog number: P6755 )
- 2-amino-2-(hydroxymethyl)-1,3-propanediol (TRIS) (DingGuo, catalog number: DH350 )
- 5,50-dithio-bis (2-nitrobenzoic acid) (DTNB) (DingGuo, catalog number: DH499 )
- Glutathione reductase (GR) (Merck KGaA, catalog number: 359960 )
- Glutathiol (GSSG) (Solarbio, catalog number: G8690 )
- Reaction solution (see Recipes)
Equipment
- Centrifuge
- Spectrophotometer
Procedure
Note: The experiment is done at room temperature (RT) except of specific indication.
- To generate a standard curve, a serial concentration of GSSG is made: 0.5, 1, 2, 5, 10, 20 μM, 2 ml for each dilution of GSSG.
- 100 μl of each GSSG standard made at step 1 is added to 3 ml of Reaction solution and incubated for 15 min. Then add 100 mM DTNB to a final concentration of 10 mM and incubate at 25 °C for 15 min.
- Measure the optical density of each sample at 412 nm by spectrophotometer, and make standard curve with a function of the concentration of GSSG standard and the optical density of each GSSG standard.
- 0.5 g of Arabidopsis leaves is ground in liquid nitrogen.
- The samples are homogenized with 1 ml extract solution and mixed completely by inverting the tube.
- The mixture is centrifuged at 10,000 x g at 4 °C for 10 min, and then the supernatant is transferred to a new tube.
- 100 μl supernatant is treated as described at step 2, and the optical density of the supernatant is measured at 412 nm as described at step 3, and calculate the GSSG content of the sample according the standard curve.
- 100 μl supernatant is mixed with 3 ml 500 mM TRIS–HCl (pH 8.0) buffer containing 10 mM DTNB and incubated at 25 °C for 15 min. The optical density is then measured at 412 nm. The Glutathione (GSH) content is determined by the same standard curve as described at step 3 with the following formula: [GSH] = 2 x [standard curve].
- The total glutathione content = [GSH] + [GSSG] (Huang et al., 2005; Chen et al., 2011)
Recipes
- Reaction solution
500 mM TRIS–HCl (pH 8.0) buffer
GR (1 U for each 3 ml reaction solution)
1 mM EDTA
3 mM MgCl2
150 μM NADPH - Extract solution
0.1% TCA (pH 2.8)
1 mM EDTA
1% (w/v) PVPP
Acknowledgments
This protocol is adapted from Huang et al. (2005) as well as other works mentioned in the reference list.
Competing interests
The authors declare no conflict of interest or competing interest.
References
- Aono, M., Kubo, A., Saji, H., Tanaka, K. and Kondo, N. (1993). Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol 34(1): 129-135.
- Baryla, A., Laborde, C., Montillet, J. L., Triantaphylides, C. and Chagvardieff, P. (2000). Evaluation of lipid peroxidation as a toxicity bioassay for plants exposed to copper. Environ Pollut 109(1): 131-135.
- Bates, L., Waldren, R. and Teare, I. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39(1): 205-207.
- Chen, L., Han, Y., Jiang, H., Korpelainen, H. and Li, C. (2011). Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J Exp Bot 62(14): 5037-5050.
- Huang, C., He, W., Guo, J., Chang, X., Su, P. and Zhang, L. (2005). Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56(422): 3041-3049.
- Inskeep, W. P. and Bloom, P. R. (1985). Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol 77(2): 483-485.
- Jin, Z. M., Wang, C. H., Liu, Z. P. and Gong, W. J. (2007). Physiological and ecological characters studies on Aloe vera under soil salinity and seawater irrigation. Process Biochem 42(4): 710-714.
- Lattanzio, V., Cardinali, A., Ruta, C., Fortunato, I. M., Lattanzio, V. M., Linsalata, V. and Cicco, N. (2009). Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ Exp Bot 65(1): 54-62.
- Madhava Rao, K. V. and Sresty, T. V. (2000). Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157(1): 113-128.
- Mandre, M., Tullus, H. and Kloseiko, J. (2002). Partitioning of carbohydrates and biomass of needles in Scots pine canopy. Z Naturforsch C 57(3-4): 296-302.
- Sibley, J. L., Eakes, D. J., Gilliam, C. H., Keever, G. J., Dozier, W. A. and Himelrick, D.G. (1996). Foliar SPAD-502 meter values, nitrogen levels, and extractable chlorophyll for red maple selections. Hort Sci 31(3): 468-470.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, Z. and Huang, R. (2013). Analysis of Malondialdehyde, Chlorophyll Proline, Soluble Sugar, and Glutathione Content in Arabidopsis seedling. Bio-protocol 3(14): e817. DOI: 10.21769/BioProtoc.817.
Category
Plant Science > Plant biochemistry > Other compound
Biochemistry > Carbohydrate > Glucose
Biochemistry > Other compound > Chlorophyll
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











