- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of IFN-α Subtype Concentrations (Virus-free, Cell-based Bioassay)
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.803 Views: 9022
Reviewed by: Fanglian HeLin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Unlocking Bio-Instructive Polymers: A Novel Multi-Well Screening Platform Based on Secretome Sampling
Shirin Fateh [...] Morgan R. Alexander
Feb 20, 2024 2668 Views

Protocol for Screening Host-Targeting Antivirals (HTAs) Using Human PBMCs and pDCs
Zhao Xuan Low [...] Pouya Hassandarvish
Mar 5, 2025 3068 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2351 Views
Abstract
The induction of type I IFN is the immediate host response against viral infections. Type I IFNs belong to a multigene family including up to 14 different IFN-α subtypes and one IFN-β. They are highly conserved and bind the same receptor (IFNAR1/2) with varying affinities, although they differ in their biological activities.
Keywords: Type I IFNsMaterials and Reagents
- 7AAD (7-amino-actinomycin D) (BD Pharmingen, catalog number: 51-68981E )
- Bovine serum albumin (BSA) (PAA Laboratories GmbH, catalog number: K41-001 )
- DMEM (Life Technologies, Gibco®, catalog number: 41966-029 )
- Superior FBS (fetal bovine serum, not heat-inactivated) (Biochrom, catalog number: S0615 )
- Mx/RAGE7 cells (virus-transformed adherent cell line with a temperature-inducible promotor; must be cultured at 32 °C; cells express the Mx transgene and a promotorless eGFP gene which is expressed due to type I IFN stimulation ) (Bollati-Fogolin and Muller, 2005)
- PBS (Life Technologies, Gibco®, catalog number: 14190-136 )
- Penicillin/streptomycin (PAA Laboratories GmbH, catalog number: P11-010 )
- Propidium iodide (eBioscience, catalog number: 00-6990-50 )
- Murine IFN-α (PBL, catalog number: 12100-1 )
- Sodium azide (Applichem, catalog number: A1430.0010 )
- Sodium pyruvate (Life Technologies, Gibco®, catalog number: 11360-039 )
- Trypsin EDTA (PAA Laboratories GmbH, catalog number: L11-004 )
- β-mercaptoethanol (Life Technologies, Gibco®, catalog number: 31350-010 )
- Media for Mx/RAGE7 cells (see Recipes)
- FACS buffer (see Recipes)
Equipment
- 96-well flat bottom plate (Falcon BD Labware, catalog number: 3072 )
- 1.5 ml microfuge tubes
- FACS tubes (BD Biosciences, Falcon®, catalog number: 352054 )
- Flow cytometer (e.g. BD LSR II)
- Incubator (37 °C; 5% CO2)
- Incubator (32 °C; 5% CO2)
Procedure
Different murine IFN-α subtypes (IFN-α1, -α2, -α4, -α5, -α6, -α9, -α11) were produced as already described (Gerlach et al., 2009).
Day 1:
- Seed Mx/RAGE7 cells in a 96 well cell culture plate (2 x 104 cells per well in 200 μl medium).
- Grow the cells for 24 h at 32 °C.
Day 2:
- Perform serial dilutions (log10) of produced IFN-α subtypes in medium in 1.5 ml tubes.
- Perform serial dilutions (log2) of recombinant IFN-α subtypes (PBL) with known concentrations from 1,000 U/ml to 31.25 U/ml (= standards) in 1.5 ml tubes.
- Decant medium of Mx/RAGE7 cells.
- Add 200 μl of the IFN-α solutions with known (standards) and unknown concentrations to the cells.
- As negative control add 200 μl of medium without IFN-α.
- Incubate the samples for 24 h at 37 °C.
Day 3:
- Decant the medium.
- Add 200 μl fresh medium to the cells.
- Incubate the samples for 48 h at 37 °C.
Day 5:
- Decant the medium.
- Wash cells with 200 μl PBS.
- Add 50 μl of trypsin EDTA (1x) 0.05% to the cells at room temperature until they suspend.
- Harvest suspended cells in FACS tubes containing 1 ml of PBS.
- Centrifuge cells (300 x g; 5 min).
- Resuspend cells with 250 μl FACS buffer.
- Add 2.5 μl 7AAD or 0.5 μl propidium iodide per sample to exclude dead cells.
- Immediately analyze cells with flow cytometer.
- IFN-α treated Mx/RAGE7 cells express eGFP (Figure 1).
- Perform standard curve with samples treated with known IFN-α concentrations (graph the data for the standard curve (Figure 2), the IFN-α titer can be determined by comparison).
- Calculate concentrations of unknown samples.
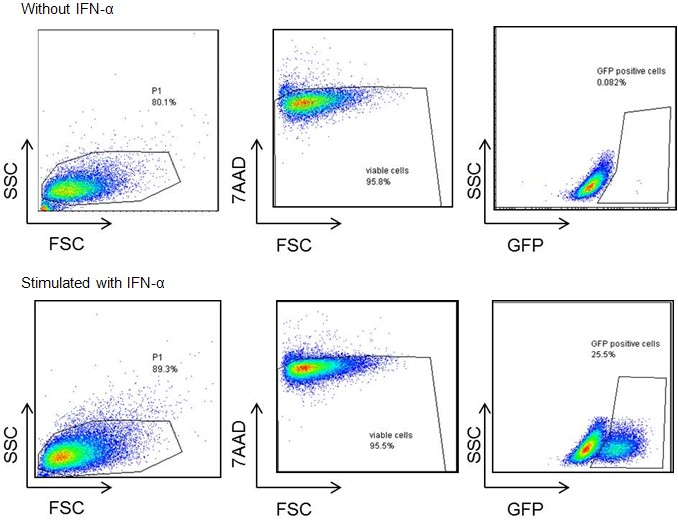
Figure 1. Representative dot plots of Mx/RAGE7 cells without IFN-α (upper panel) and with IFN-α (lower panel)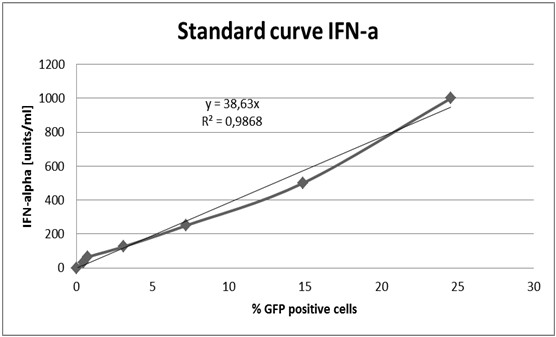
Figure 2. Standard curve of IFN-αRecipes
- Media for Mx/RAGE7 cells
DMEM
10% FBS
1 mM sodium pyruvate
1% penicillin/streptomycin
50 μM β-mercaptoethanol - FACS buffer
PBS
0.1% BSA
0.02% sodium azide
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (GRK 1045).
References
- Bollati-Fogolin, M. and Muller, W. (2005). Virus free, cell-based assay for the quantification of murine type I interferons. J Immunol Methods 306(1-2): 169-175.
- Gerlach, N., Gibbert, K., Alter, C., Nair, S., Zelinskyy, G., James, C. M. and Dittmer, U. (2009). Anti-retroviral effects of type I IFN subtypes in vivo. Eur J Immunol 39(1): 136-146.
- Gibbert, K., Joedicke, J. J., Meryk, A., Trilling, M., Francois, S., Duppach, J., Kraft, A., Lang, K. S. and Dittmer, U. (2012). Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Pathog 8(8): e1002868.
- Media for Mx/RAGE7 cells
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gibbert, K. (2013). Measurement of IFN-α Subtype Concentrations (Virus-free, Cell-based Bioassay). Bio-protocol 3(12): e803. DOI: 10.21769/BioProtoc.803.
- Gibbert, K., Joedicke, J. J., Meryk, A., Trilling, M., Francois, S., Duppach, J., Kraft, A., Lang, K. S. and Dittmer, U. (2012). Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Pathog 8(8): e1002868.
Category
Microbiology > Microbe-host interactions > Virus
Immunology > Immune cell function > Cytokine
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link