- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Growth Assay and Detection of TRP and Indole Derivatives in Piriformospora indica Culture Supernatant by LC-MS/MS
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.800 Views: 13143

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Salicylic Acid (SA) and SA-glucosides in Arabidopsis thaliana
Valérie Allasia [...] Harald Keller
May 20, 2018 13141 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1582 Views
Abstract
The mutualistic root endophyte Piriformospora indica colonizes a wide range of plants and the colonization of root cells by this fungus is very often associated with beneficial effects to its host, such as growth promotion and increased biotic and abiotic stress tolerance. These traits may be based on general mechanisms and signaling pathways common to many different plant species. One such mechanism could be the recruitment of phytohormone pathways by P. indica. It is known, that many mutualistic microorganisms are able to synthesize and secrete phytohormones during the interaction with their host plants. This protocol has been successfully utilized to analyze tryptophan (TRP)-dependent biosynthesis of indole-3-acetic acid (IAA) and its indole derivatives by P. indica as well as their influence on the growth of this fungus (Hilbert et al., 2012).
Materials and Reagents
- Indole derivative:
TRP (Sigma-Aldrich, catalog number: T0254-500g )
IAD (indole-3-acetaldehyde) (Sigma-Aldrich, catalog number: I1000-100mg )
IAA (Sigma-Aldrich, catalog number: I5148-2g ) - Neubauer improved counting chamber (Marienfeld-Superior)
- Sterile scalpel
- Sterile drigalski spatula
- Miracloth filter 15 cm x 15 cm (Merck KGaA, catalog number: 475855 )
- Ruler
- 0.3 ml polypropylene snap ring microvials
- 0.9% NaCl
- 0.002% (v/v) Tween water 20
- 90% methanol (HPLC grade)
- Acetonitrile (HPLC grade)
- Acetic acid (HPLC grade)
- Chlamydospores solution
- Microelements (MnCl2.4H2O, H3BO3, ZnSO4.7H2O, KI, Na2MO4.2H2O, CuSO4.5H2O)
- Glucose
- Peptone
- Yeast extract
- Casamine acids
- Agar
- Complete medium (CM medium) (see Recipes)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
Equipment
- 1.5 ml Eppendorf tubes
- 50 ml Falcon tube
- Petri dishes (12 mm diameter)
- Incubator (e.g. B. Braun Biotech International, Certomat BS-1)
- Biofuge Primo R (Heraeus, Germany) (Rotor, catalog number: 7590 )
- Phenomenex Luna 250 x 4.6 mm C18 RP-HPLC column (Phenomenex)
- Phenomenex Luna 10 x 4.6 mm C18 RP-HPLC guard column (Phenomenex)
- ICS3000 HPLC system (Dionex)
- QTrap 3200 triple quadrupole mass spectrometer (Applied Biosystems SCIEX)
- Polypropylene snap ring microvial
Procedure
- Growth Assay
- Collect spores from 3 to 4 weeks old Piriformospora indica plate cultures (see Figure 1).
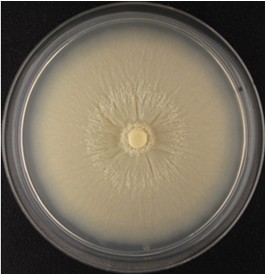
Figure 1. Four-week-old P. Indica agar plate- Pour approximately 5 ml sterile 0.002% Tween water 20 on 3-4 weeks old P. indica plate under sterile condition at room temperature (RT).
- Scratch plate with sterile Drigalski spatula and/or scalpel and mix.
- Pour spore solution through miracloth filter and collect flow through in 50 ml Falcon tube.
- Centrifuge for 7 min at 3,500 rpm discard supernatant.
- Wash pellet with 5-10 ml 0.002% Tween water 20.
- Centrifuge for 7 min at 3,500 rpm, discard supernatant.
- Wash pellet with 5-10 ml 0.002% Tween water 20.
- Centrifuge for 7 min at 3,500 rpm, discard supernatant.
- Resuspend spore pellet in 10 ml 0.002% Tween water 20, count spores with counting chamber (e.g. Neubauer improved) and dilute to requested spore concentration (e.g. 500,000 spores/ml)
- Pour approximately 5 ml sterile 0.002% Tween water 20 on 3-4 weeks old P. indica plate under sterile condition at room temperature (RT).
- Inoculate 50 ml CM medium (Pham et al., 2004) supplemented with appropriate indole derivative (e.g. 2.5 mM TRP; 250 μM IAD or 1, 10, 100 μM IAA) with 400 μl chlamydospores solution (500,000 spores/ml) and cultivate for 7 days at 28 °C in the dark (alternatively wrap flasks with aluminium foil). Use mock inoculated flask as a negative control.
- Separate supernatant from mycelium using miracloth filter (check the mass of each filter before).
- Wash mycelium with 0.9% NaCl and let the whole miracloth filter with fungal biomass dry overnight in oven (85 °C).
- Measure the dry fungal biomass (= mass of miracloth filter with dried fungal biomass – mass of empty miracloth filter).
- Place 5 mm agar plugs from the 3 to 4 weeks old Piriformospora indica plate culture in the middle of a CM agar plate supplemented with the appropriate indole derivative (2.5 mM TRP, 250 μM IAD or 1, 10, 100 μM IAA). Use CM agar plate as control.
- Use ruler to measure colony diameter after 14 days of cultivation at 28 °C in the dark.
- Collect spores from 3 to 4 weeks old Piriformospora indica plate cultures (see Figure 1).
- Detection of tryptophan and indole derivatives in culture supernatant by LC-MS/MS
- Use a 15 μl aliquot of P. indica culture supernatant obtained from section I point 2 of the procedure.
- Add 1 ml of 90% methanol.
- Vortex briefly and dilute an aliquot 1:10 in 90% methanol into a 0.3 ml polypropylene snap ring microvial.
- Analyze 10 μl of the 1:10 dilution by LC-MS/MS.
- IAA and ILA are separated on an ICS3000 HPLC system equipped with a Phenomenex Luna 250 x 4.6 mm C18 RP-HPLC column with the following gradient
- 0 to 5 min hold at 80% buffer A, 20% buffer B
- 5 to 26 min hold at 54% buffer A, 46% buffer B
- 26 to 27 min ramp to 10% buffer A, 90% buffer B
- 32 to 34 min ramp to 80% buffer A, 20% buffer B
- 32 to 34 min ramp to 80% buffer A, 20% buffer B
- 34 to 45 min equilibrate with 80% buffer A, 20% buffer B
- Subject the HPLC eluate to coupled electrospray ionization in the negative ionization mode and to subsequent tandem MS analysis on the QTrap 3200 mass spectrometer with the following settings:
- dwell time 75 ms
- declustering potential (DP) -22 V (IAA), -30 V (ILA)
- entrance potential (EP) -7 V (IAA), -55 V (ILA)
- collision energy (CE) -15 V (IAA), -18 V (ILA)
- collision energy (CE) -15 V (IAA), -18 V (ILA)
- collision cell exit potential (CXP) 0 V (IAA), -4 V (ILA)
- Quantitate IAA using the m/z transitions 174/130 and 174/128.
- Quantitate ILA using the m/z transitions 204/128 and 204/158.
- Employ commercially available authentic substances as references.
- Use a 15 μl aliquot of P. indica culture supernatant obtained from section I point 2 of the procedure.
Recipes
- Complete medium (CM medium; Pham et al., 2004)
- CM medium (1 L)
50 ml 20x salt solution
20 g glucose
2 g peptone
1 g yeast extract
1 g casamine acids
1 ml microelements
15 g agar - 20x salt solution
120 g NaNO3
10.4 g KCl
10.4 g MgSO4.7H2O
30.4 g KH2PO4 - Microelements
6 g MnCl2.4H2O
1.5 g H3BO3
2.65 g ZnSO4.7H2O
750 mg KI
2.4 mg Na2MO4.2H2O
130 mg CuSO4.5H2O
- CM medium (1 L)
- Buffer A
0.75% acetic acid, pH 2.55 (adjust with acetic acid, if necessary) - Buffer B
acetonitrile/0.75% acetic acid, pH 2.55 (adjust with acetic acid, if necessary)
Acknowledgments
This protocol is adapted from Hilbert et al. (2012).
References
- Hilbert, M., Voll, L. M., Ding, Y., Hofmann, J., Sharma, M. and Zuccaro, A. (2012). Hilbert, M., Voll, L. M., Ding, Y., Hofmann, J., Sharma, M. and Zuccaro, A. (2012). Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol 196(2): 520-534.
- Pham,G. H., Singh, A., Malla, R., Kumari, R., Prasad, R., Sachdev, M., Luis, P., Kaldorf, M., Peskan, T., Herrmann, S. (2004). Interaction of P. indica with other microorganisms and plants. In: Varma A, Abbott L, Werner D, Hampp R, eds. Plant Surface Microbiol Heidelberg, Germany: Springer, 237-265.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hilbert, M., Voll, L. M., Hofmann, J. and Zuccaro, A. (2013). Growth Assay and Detection of TRP and Indole Derivatives in Piriformospora indica Culture Supernatant by LC-MS/MS. Bio-protocol 3(12): e800. DOI: 10.21769/BioProtoc.800.
Category
Plant Science > Plant physiology > Endosymbiosis
Microbiology > Microbe-host interactions > Fungus
Biochemistry > Other compound > Plant hormone > Indole
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









