- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Hairy Root Transformation in Lotus japonicus
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.795 Views: 20280

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Agrobacterium-mediated Transformation of Japonica Rice Using Mature Embryos and Regenerated Transgenic Plants
Ammar Elakhdar [...] Takahiko Kubo
Sep 20, 2021 6237 Views

Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Alka Srivastava [...] Praveen C. Verma
May 20, 2023 4357 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1979 Views
Abstract
In L. japonicus, hairy root transformation is the very useful technique to generate transformed root systems in a short term. This protocol was previously described (Kumagai and Kouchi, 2003) with some modifications. After the infection of Agrobacterium rhizogenes, L. japonicus develops not only transformed but also untransformed roots. Thus, transgenic roots need to be identified by certain indications. In this protocol, we use the GFP florescent signals as such indication.
Materials and Reagents
- Germination plate (1% agar in sterilized water)
- B5 salt
- Agar
- Sucrose
- Gamborg’s vitamin solution (Sigma-Aldrich, catalog number: G1019 )
- Meropen (Dainippon Sumitomo Pharma)
- LB medium
- Sterilized water
- Co-cultivation medium (see Recipes)
- Hairy root elongation medium (see Recipes)
Equipment
- Clean bench
- L. japonicus growth facility
- Surgical knife
- Sterilized dish (9 cm in diameter)
- Sterilized filter paper (7-8 cm in diameter)
- Sterilized square dish (10 x 14 cm)
Procedure
A. Plant growth
- Sandpaper the surface of L. japonicus Gifu or MG-20 seeds, and then incubate them in 2% sodium hypochlorite solution for 5 min. Wash the seeds several times with sterilized water and incubate the seeds overnight in the sterilized water.
- Surface-sterilized seeds are germinated and grown in the germination plate.
- Place the plate vertically in a growth cabinet (For Gifu, 23 °C 24 h dark for first 3 days and 23 °C 16 h light/8 h dark for next 2 days; For MG-20, 23 °C 24 h dark for first 2 days and 23 °C 16 h light/8 h dark for next 2 days).
B. Culture of Agrobacterium
Streak A. rhizogenes harboring the desired construct on LB plate with appropriate antibodies for 2 days at 28 °C, then spread bacteria all around sterilized dish (9 cm in diameter) and incubate for 1 day.
C. Infection of A. rhizogenes with L. japonicus
- Collect the bacteria with bacteria spreader from LB plate and suspend 6 ml sterilized water.
- Set a sterilized filter paper in a new dish, and saturate it with bacterial suspension by pipetting.
- Place the juvenile plants on the saturated filter paper, and cut at the middle of the hypocotyl with surgical knife (Figure 1).
- Transfer the seedlings of shoot side onto co-cultivation media (cut end is need to be about 1 mm in depth from agar surface), and place the plate horizontally in a growth cabinet (23 °C 24 h dark) for 1 day.
- Place the plate vertically and incubate at 23 °C (16 h light/8 h dark) for 5 days.
D.Induction of hairy roots
- Transfer the plants onto hairy root elongation media and incubate vertically in a growth cabinet (23 °C 16 h light/8 h dark).
- After 10-14 days, the hairy roots should be approximately 2-5 cm in length. Pick up plants with transgenic roots expressing florescent proteins for further analysis (Figure 1).
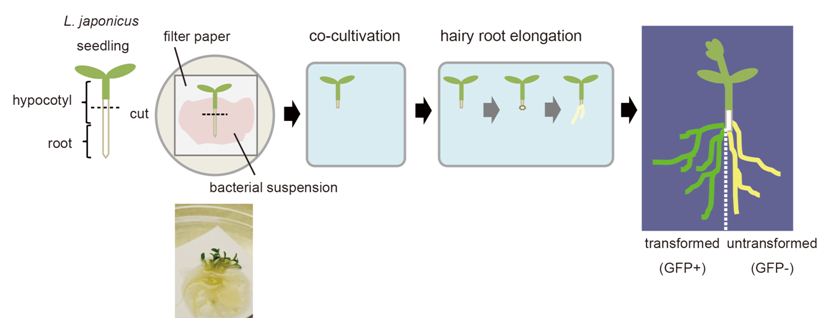
Figure 1. Hairy root transformation in L. japonicus
Recipes
- Co-cultivation medium
1/2x B5 salt
1/2x Gamborg’s vitamin solution
1% agar
Maintain pH with KOH at pH 5.5, pour into a square dish
Mix all components except for Gamborg’s vitamin solution, and autoclave the mixture, and then add Gamborg’s vitamin solution. - Hairy root elongation medium
1/2x B5 salt
1/2x Gamborg’s vitamin solution
12.5 μg/ml meropen
1% sucrose
1% agar
Maintain pH with KOH at pH 5.5, pour into a square dish
Mix all components except for Gamborg’s vitamin solution and meropen, and autoclave the mixture, and then add Gamborg’s vitamin solution and meropen.
Acknowledgments
This work was supported by MEXT/JSPS KAKENHI, Japan (22870035, 23012038, 25114519 to Takuya Suzaki).
References
- Kumagai, H. and Kouchi, H. (2003). Gene silencing by expression of hairpin RNA in Lotus japonicus roots and root nodules. Mol Plant Microbe Interact 16(8): 663-668.
- Okamoto, S., Ohnishi, E., Sato, S., Takahashi, H., Nakazono, M., Tabata, S. and Kawaguchi, M. (2009). Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol 50(1): 67-77.
- Suzaki, T., Yano, K., Ito, M., Umehara, Y., Suganuma, N. and Kawaguchi, M. (2012). Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139(21): 3997-4006.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Okamoto, S., Yoro, E., Suzaki, T. and Kawaguchi, M. (2013). Hairy Root Transformation in Lotus japonicus. Bio-protocol 3(12): e795. DOI: 10.21769/BioProtoc.795.
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > Transformation
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








