- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring Stomatal Density in Rice
Published: Vol 3, Iss 9, May 5, 2013 DOI: 10.21769/BioProtoc.753 Views: 20369
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Actin Organization and Quantification in Fixed Arabidopsis Pollen Grains and Tubes
Xiaolu Qu [...] Shanjin Huang
Jan 5, 2020 5354 Views

Targeting Ultrastructural Events at the Graft Interface of Arabidopsis thaliana by A Correlative Light Electron Microscopy Approach
Clément Chambaud [...] Lysiane Brocard
Jan 20, 2023 2981 Views

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2322 Views
Abstract
The number of stomata on leaves is known to be affected by various environmental factors and intrinsic developmental program. Stomatal density and stomatal index are generally used as indicators of the leaf development and the plant growth. This protocol describes an easy, non-destructive method for preparing imprints of the rice leaf surface that is suitable for observation and counting of stomata. Researchers can process many leaf samples at once in the field or in the green house distance from the laboratory.
Keywords: RiceMaterials and Reagents
- Microscope cover glasses (Matsunami Glass, 24 x 40 mm No.1)
Note: Though tougher glass slides (1-1.2 mm thickness) are safer, you should use cover glasses if the objective lens of the microscope is designed for standard 0.17 mm glass thickness. Only special objective lenses that have long working distances (ELWD, LWD) will allow observation through a thick glass slide. Alternatively objectives designed for use without a cover glass (NCG, NC) will enable direct observation of imprints placed on the slide glasses. I use large (24 x 40 mm) glasses for making imprints covering large leaf area. Larger glass is also useful when you set it on the microscope stage. - Instant glue (Aron Alpha Super Set) (Toagosei Co., catalog number: EA936A-5 )
Note: Aron Alpha Super Set includes liquid glue and accelerator. Aron Alpha is sold as Krazy Glue in North America and Cyanolit in Europe. - Accelerator for instant glue
Note: While I use the genuine accelerator made by Toagosei, third-party accelerators developed for Aron Alpha (Krazy Glue) will work fine. You can observe stomata without the accelerator, but the imprints may be less clear.
Equipment
- Light microscope with equipment for photomicrography
- Eyepiece micrometer and stage micrometer
Note: These are indispensable for measurement of field of view unless your microscope can automatically calculate scale bars.
Procedure
- Select the leaves for observation. Make sure that they are not wet with rain or dew (Figure 1). Imprints can be taken from both upper (adaxial) and lower (abaxial) surfaces. Unless there are particular reasons, widest (middle) region of mature leaf blade should be selected as a target area. Avoid thick major vain for making smooth imprints.

Figure 1. Typical healthy leaf of rice.
- Apply several drops of the accelerator to the cover glass (Figure 2) and wait until they are thoroughly dry (2-3 min, at room temperature) (Figure 3).
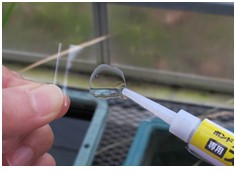
Figure 2. Place a drop of accelerator on a cover glass.
Figure 3. Dried accelerator on the cover glass. - Immediately after applying a drop of instant glue to the surface of the leaf, press the accelerator side of the cover glass on the leaf for about 30 seconds (Figures 4 and 5).

Figure4. Instant glue dropping on the leaf surface.
Figure 5. Drying glue mixture. - Remove the cover glass from the leaf gently. Make sure that the imprint is on the cover glass (Figure 6). If glue mixture is completely dried, obtained imprint will be sturdy and durable. When a healthy leaf is used, only a remnant will be left on the leaf surface.

Figure 6. Removed imprints on the cover glasses (left).
Figure 7. A cover glass placed on the microscope stage. In this case, imprint is placed on the upper surface of the cover glass for imaging on inverted microscope. - Observe imprints under the light microscope (Figure 7). Stomata in rice are formed in rows or files that are parallel to the sides of the leaf (Figure 8) (Hoshikawa, 1989). Therefore, finding stomata is relatively easy. Take photographs of the magnified image, calculate captured leaf area by using the micrometer or by the build-in imaging software, and then count all stomata within the printed image. I routinely counted stomata within a 0.42 mm2 of leaf area.
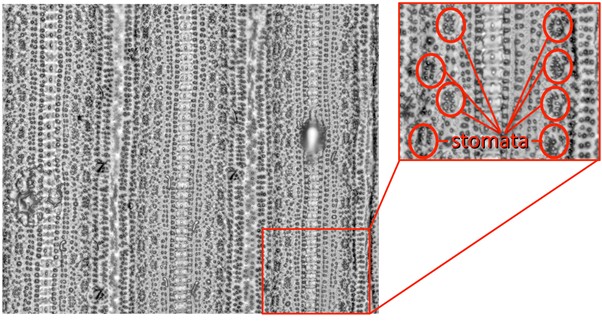
Figure 8. Typical imprint image of medial leaf reagion. Red circles indicate positions of stomata.
Note: Since imaging through a microscope gives a very shallow depth of field, only a very narrow region of the picture may be in focus at a time. To solve the focus problem, you can use autofocusing microscope or photo-processing software. I routinely use Keyence BZ-9000 microscope with optical software (Keyence, Osaka, Japan). ImageJ software (http://rsb.info.nih.gov/ij/) with appropriate plug-ins (e.g. Stack Focuser, Extended Depth of Field) also generates reasonable in-focus composite images Figure 9).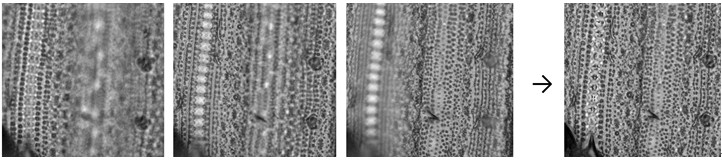
Figure 9. Focus stacking. Left are the three source images at different focal depths. Right is a composite image generated by ImageJ and the Stack Focuser plug-in (http://rsb.info.nih.gov/ij/plugins/stack-focuser.html). - Imprints can be stored without any sealing treatment for long time at room temperature. I could get clear images even after 2 years.
Acknowledgments
I am grateful to Ryoko Kaji for her technical assistance. This work was supported by the Kyushu University Interdisciplinary Programs in Education and Projects in Research Development (P&P), a Grant-in-Aid for Scientific Research on Innovative Areas (No. 21114002) and the Ministry of Education, Science and Culture of Japan (No. 22570045).
References
- Hoshikawa, K. (1989). The growing rice plant: an anatomical monograph. Tokyo: Nobunkyo xvi, 310p.-illus.. ISBN 245913836.
- Kusumi, K., Hirotsuka, S., Kumamaru, T. and Iba, K. (2012). Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot 63(15): 5635-5644.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kusumi, K. (2013). Measuring Stomatal Density in Rice. Bio-protocol 3(9): e753. DOI: 10.21769/BioProtoc.753.
Category
Plant Science > Plant physiology > Tissue analysis
Plant Science > Plant cell biology > Cell structure
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










