- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Lipid Transfer Assay
Published: Vol 3, Iss 9, May 5, 2013 DOI: 10.21769/BioProtoc.691 Views: 13122

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Superoxide Dismutase (SOD) and Catalase (CAT) Activity Assay Protocols for Caenorhabditis elegans
Jing Zhang [...] Lili Xue
Aug 20, 2017 38853 Views

An Optimized Enzyme-Coupled Spectrophotometric Method for Measuring Pyruvate Kinase Kinetics
Saurabh Upadhyay
Aug 20, 2025 2465 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 505 Views
Abstract
This is a protocol to detect lipid transfer activity of NRF-5, a member of the LPS binding/lipid transfer protein family. The lipid transfer activity is examined by using isotope-labeled cholesterol and liposomes, and tested in two directions (Figure 1): from proteins to liposomes and from liposomes to proteins.
Materials and Reagents
- PC (Avanti-Polar Lipids)
- PE (Avanti-Polar Lipids)
- Cholesterol (Avanti-Polar Lipids, catalog number: 700000 )
- 1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine (DOPE) (Avanti, catalog number: 850725 )
- 1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (DOPC) (Avanti, catalog number: 770375 )
- 1 mCi (37 MBq) (PerkinElmer, catalog number: NET139001MC )
- Protein of interest tagged with Flag
- Tris-HCl (pH 7.4)
- NaCl
- Anti-Flag M2 agarose beads (Sigma-Aldrich, catalog number: A2220 )
- Flag peptide (Sigma-Aldrich, catalog number: F3290 )
- Chloroform
- Wash buffer (see Recipes)
- Elution buffer (see Recipes)
Equipment
- Avanti Mini-Extruder (Avanti, catalog number: 610023 )
- Vortexer
- Centrifuges
- Rotator
- Branson tip-sonicator (Cole-parmer Cp750)
- Scintillation counter (Wallac MicroBeta TriLux, catalog number: 1450-023 )
Procedure
- Preparation of liposomes (room temperature)
- Liposome in the absence of cholesterol is made by Avanti Mini-Extruder at room temperature. The dried 1.25 mg mix of PC (75%) and PE (25%) is hydrated in 1 ml buffer (50 mM Tris-Cl, 150 mM NaCl). 100 nm unilamellar vesicles are obtained by extrusion as described:
(http://www.avantilipids.com/index.php?option=com_content&view=article&id=185&Itemid=193) - Liposomes containing [3H]cholesterol are generated by using a standard sonication procedure.
- Dissolve dried 2.5 mg mix of PC (75%) and PE (25%) in 200 μl chloroform by vortex.
- Take 8 μl of above chloroform dissolved lipid mix and add 0.001 mg [3H]cholesterol (~2% molar mass).
- The chloroform is evaporated and dried under a stream of nitrogen. Longer drying time (4-12 h) can be used to remove any trace of organic solvent.
- The dry lipid film is hydrated by adding 0.5 ml of buffer (50 mM Tris-Cl, 150 mM NaCl). After vortex at room temperature for 20 min, the large multilamellar vesicle suspension is disrupted with a Branson tip-sonicator until the suspension clear. For sonication, the samples are placed on ice and sonicated for 10 min with cycles including 9 seconds sonication, 9 seconds interval and 35% input.
- Metal particles from the sonicator tip and undisrupted lipid aggregates are removed by centrifugation at 100,000 x g for 30 min at 4 °C. The resulting hazy supernatant, composed primarily of small unilamellar vesicles, is stored at 4 °C. The liposomes can be stored at this condition for one week.
- Dissolve dried 2.5 mg mix of PC (75%) and PE (25%) in 200 μl chloroform by vortex.
- Liposome in the absence of cholesterol is made by Avanti Mini-Extruder at room temperature. The dried 1.25 mg mix of PC (75%) and PE (25%) is hydrated in 1 ml buffer (50 mM Tris-Cl, 150 mM NaCl). 100 nm unilamellar vesicles are obtained by extrusion as described:
- Examine [3H]Cholesterol transfer from liposomes to proteins (Figure 1)
Reactions are performed on ice.- In the [3H]cholesterol/liposome to protein transfer assay, each reaction contains, in a final volume of 200 μl buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4), 4 μg of PC:PE:[3H]cholesterol liposomes, and different amounts of the acceptor protein EGFP-FLAG and EGFP::NRF-5-FLAG(15 and 30 μg) purified from 293T cells (Zhang et al., 2012). EGFP-FLAG is used as the negative control.
- After incubation for 30 min at 4 °C, each mixture is diluted with 600 μl 50 mM Tris-Cl, 150 mM NaCl, followed by adding 70 μl 50% Flag beads.
- After incubation for about 1 h at 4 °C on shaker, the Flag beads are washed 4-5 times in 1,000 μl wash buffer on shaker, and bounded [3H]cholesterol is quantified by scintillation counting.
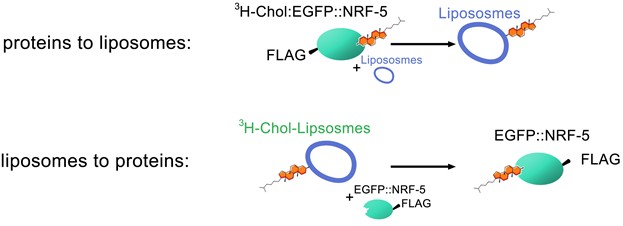
Figure 1. Schematic diagrams of the lipid transfer assay in two directions
- In the [3H]cholesterol/liposome to protein transfer assay, each reaction contains, in a final volume of 200 μl buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4), 4 μg of PC:PE:[3H]cholesterol liposomes, and different amounts of the acceptor protein EGFP-FLAG and EGFP::NRF-5-FLAG(15 and 30 μg) purified from 293T cells (Zhang et al., 2012). EGFP-FLAG is used as the negative control.
- Examine [3H]Cholesterol transfer from proteins to liposomes (Figure 1)
- Protein-[3H]cholesterol complex is obtained by incubating EGFP-FLAG or EGFP::NRF-5-FLAG (400 pmol) with [3H]cholesterol (100 pmol) in a final volume of 300 μl buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4) for 3 h at 4 °C. The protein-[3H]cholesterol complex is pulled down by incubating with 100 μl 50% Flag beads for 2 h at 4 °C, washing 6 times as above and eluted with 100 μl Flag peptide (100 mg ml-1) for two times.
- In the protein-to-liposome transfer assay, each reaction contains, in a final volume of 200 μl buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4), [3H]cholesterol complexed to either EGFP or EGFP-NRF-5 (40 μl), and different amounts of acceptor PC liposomes (50 and 100 ng).
- After incubation for 30 min at 4 °C, each mixture is diluted with 600 μl of buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4).
- Liposomes are separated by centrifuging at 10,000 x g (hard to detect weight at this stage) for 30 min at 4 °C. The lipososomes can be seen as a small white patch at the bottom of the tube.
- Wash the liposomes 4-5 times in the buffer (50 mM Tris-HCl, 150 mM NaCl), with 500 μl buffer used in each tube at each time. The liposomes are collected by centrifuge (10,000 x g) after each wash. The amount of [3H]cholesterol transferred to liposomes is determined by scintillation counting.
- Protein-[3H]cholesterol complex is obtained by incubating EGFP-FLAG or EGFP::NRF-5-FLAG (400 pmol) with [3H]cholesterol (100 pmol) in a final volume of 300 μl buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.4) for 3 h at 4 °C. The protein-[3H]cholesterol complex is pulled down by incubating with 100 μl 50% Flag beads for 2 h at 4 °C, washing 6 times as above and eluted with 100 μl Flag peptide (100 mg ml-1) for two times.
Recipes
- Wash buffer
50 mM Tris-Cl, 150 mM NaCl (pH 7.4) - Elution buffer
50 mM Tris-Cl, 150 mM NaCl (pH 7.4), Flag peptide
Acknowledgments
This protocol is adapted from Zhang et al. (2012) and Infante et al. (2008).
References
- Infante, R. E., Wang, M. L., Radhakrishnan, A., Kwon, H. J., Brown, M. S. and Goldstein, J. L. (2008). NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A 105(40): 15287-15292.
- Zhang, Y., Wang, H., Kage-Nakadai, E., Mitani, S. and Wang, X. (2012). C. elegans secreted lipid-binding protein NRF-5 mediates PS appearance on phagocytes for cell corpse engulfment. Curr Biol 22(14): 1276-1284.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, Y. and Wang, X. (2013). In vitro Lipid Transfer Assay. Bio-protocol 3(9): e691. DOI: 10.21769/BioProtoc.691.
Category
Biochemistry > Protein > Activity
Biochemistry > Lipid > Lipid transport
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









