- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Primary Culture of Cortical Neurons
Published: Vol 3, Iss 8, Apr 20, 2013 DOI: 10.21769/BioProtoc.496 Views: 26723
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3937 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Primary culture of neurons from cerebral cortex is a popular model to study neuronal function in vitro and to explore the molecular mechanism of neurite outgrowth in the developing and adult central nervous system. This protocol is for preparing a culture of cerebral cortical neurons from postnatal rodent brain (Muramatsu et al., 2012). One day after cell plating, we can observe neurite outgrowth by microscope.
Keywords: BrainMaterials and Reagents
- Poly-L-lysine (Sigma-Aldrich, catalog number: P2636 )
- Dulbecco's Modified Eagle Medium, DMEM (Life Technologies, InvitrogenTM, catalog number: 12800-017 )
- Fetal bovine serum (Life Technologies, Gibco®, catalog number: 10437 )
- 2.5% Trypsin solution (Life Technologies, Gibco®, catalog number: 15090-046 )
- DNase (Sigma-Aldrich, catalog number: DN25 )
- Penicillin/streptomycin (Life Technologies, Gibco®, catalog number: 15140 )
- NaH2PO4.2H2O
- Na2HPO4.12H2O
- NaCl
- DMEM
- FBS
- Serum-containg culture medium (see Recipes)
- PBS (see Recipes)
Equipment
- Cell culture incubator
- Centrifuge
- Stereoscopic microscope
- Scissors, forceps, and knives
- Sterile filter (0.45 μm)
- Cell strainer 70 μm nylon (BD Biosciences, catalog number: 352350 )
- 24 well culture plate
Procedure
- Coating and washing culture plates.
- Dilute poly-L-lysine stock solution with sterile PBS to 100 μg/ml. Add optimal volume of solution to each well of culture plates. In case of 24 well culture plate, 0.5 ml solution is enough. Coating allows neurons to adhere to the culture plates.
- Leave the plates in incubator for at least 1 h. After that, wash the wells twice with 1 ml/well of sterile PBS. Completely remove the PBS before use. It is not necessary to dry the plate after PBS washing.
- Dilute poly-L-lysine stock solution with sterile PBS to 100 μg/ml. Add optimal volume of solution to each well of culture plates. In case of 24 well culture plate, 0.5 ml solution is enough. Coating allows neurons to adhere to the culture plates.
- Choose appropriate anesthesia according to the recommendations of your Institutional Animal Care and Use Committee. After anesthetization, cut the scalp and remove the skull. We use postnatal day 1 mice to measure the length of corticospinal neuron. Pick up whole brain from pups and place it in cold PBS on ice (Chen et al., 2007).
- Under a microscope, dissect out the cerebral cortex of brains. Remove meninges and blood vessels. The tissues are cut into as small piece (1 mm cubes) as can with sterile knives.
- Transfer tissues to a 50 ml tube. The tissues are trypsinized in 10 ml of the trypsin solution (0.25% trypsin, 100 μg/ml DNase in PBS), and are incubated at 37 °C for 15 min.
- Add an equal amount of 10% FBS-DMEM, and pipettes up and down a few times to help with tissues dissociation.
- Centrifuge at 300 x g for 3 min at room temperature, discard the supernatant, and resuspend the pellet in fresh DMEM (not 10% FBS-DMEM).
- The cells are filtered through a 70 μm nylon cell strainer to obtain single cell suspension. Centrifuge the resulting cell suspension at 300 x g for 3 min at room temperature. Resuspend the cell pellet in fresh DMEM (not 10% FBS-DMEM).
- Count the number of cells and adjust the concentration using 10% FBS-DMEM. To measure the neurite length, plate the cells on a 4-well chamber slide at a density of 5 x 104 cells/well. Transfer the cell suspension to culture plates and incubate for 24 h at 37 °C with 5% CO2. To investigate the effect of pharmacological agents, change to the medium containing each agent 2 h after cell plating. Representative image shows the neuronal class III β-Tubulin (Tuj-1) labeled cortical neuron obtained 1 day culture.
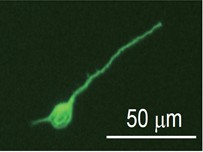
Figure 1. Image of cultured cortical neuron. Representative image shows the neuronal class III β-Tubulin (Tuj-1) labeled cortical neuron obtained 1 day culture.
Recipes
- Poly-L-lysine: Dissolve of sterile distilled water to make stock solution (10 mg/ml). Keep aliquots at -20 °C. Dilute with sterile PBS right before use.
- Serum-containg culture medium
Reagents Volume DMEM 445 ml FBS 50 ml Penicillin/streptomycin 5 ml - PBS
Reagents Volume NaH2PO4·2H2O 0.312 g Na2HPO4·12H2O 2.8652 g NaCl 8.5 g DW 1 L
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (A) (25710006) from the Japan Society for the Promotion of Sciences to RM, the Osaka University Program for the Support of Networking among Present and Future Researchers to RM, and a Grant-in-Aid for Scientific Research (S) from JSPS (25221309) to TY.
References
- Chen, Y., Balasubramaniyan, V., Peng, J., Hurlock, E. C., Tallquist, M., Li, J. and Lu, Q. R. (2007). Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protocols 2(5): 1044-1051.
- Muramatsu, R., Takahashi, C., Miyake, S., Fujimura, H., Mochizuki, H. and Yamashita, T. (2012). Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med 18(11): 1658-1664.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Muramatsu, R. and Yamashita, T. (2013). Primary Culture of Cortical Neurons. Bio-protocol 3(8): e496. DOI: 10.21769/BioProtoc.496.
Category
Neuroscience > Development > Neuron
Cell Biology > Cell isolation and culture > Cell differentiation
Neuroscience > Neuroanatomy and circuitry > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link