- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fusarium Virulence Assay on Wheat and Barley Seedlings
Published: Vol 3, Iss 7, Apr 5, 2013 DOI: 10.21769/BioProtoc.446 Views: 14032
Reviewed by: Fanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect
Ben Niu and Roberto Kolter
Jun 20, 2018 10501 Views

Tracking Root Interactions System (TRIS) Experiment and Quality Control
Hassan Massalha [...] Asaph Aharoni
Apr 20, 2019 7343 Views

A Quick Method for Screening Biocontrol Efficacy of Bacterial Isolates against Bacterial Wilt Pathogen Ralstonia solanacearum in Tomato
Heena Agarwal [...] Niraj Agarwala
Nov 20, 2020 5558 Views
Abstract
Fusarium root and crown rot is a very important complex disease of small grain cereals worldwide which may lead to very high yield losses. Traditional virulence assays are time consuming and often require plants to be grown in greenhouses or climatic chambers in soil. We describe a rapid laboratory assay for assessing such a disease in wheat and barley seedlings. The method could be successfully used for testing fungal virulence as well as to assess plant resistance.
Materials and Reagents
- Fungal strains
- 25% Campbell`s V8 juice (pH 6.5 with NaOH)
- 1.2% agar plates (9 cm diameter)
- Wheat and barley seeds
- Sterilizing solution (0.64% sodium hypochlorite - 10% ethanol)
- Sterile deionized water
Equipment
- Petri dishes (9 and 14 cm diameter)
- Filter paper (Whatman No.3, 8 and 12.5 cm diameter)
- Pipette tip or cork borer
- Sealing film (Phyto Technology Laboratories) or parafilm
- Ruler
- Razor blades
- Laminar flowhood
- Illuminated incubator at 21 ± 1 °C, light intensity > 90 μmols
- Fungal growth cabinet with white (one 18 W lamp) and black fluorescent (one 18 W lamp) lights on 12/12 h day/night cycle at 20-22 °C
- Incubator
Procedure
- Day 1
Grow fungal colonies on V8 agar plates for 7 days at 20-22 °C under white and black fluorescent light on a 12/12 h day/night cycle, starting from stored fungal cultures or colonies from plates. - Day 3 (wheat) or 4 (barley)
For each fungal isolate or plant genotype 16 germinated seeds are required. If testing fungal isolate virulence, a mock treatment is also required. Barley is more vigorous in the early stages of growth and seeds are allowed to germinate for 2 days whereas wheat is allowed to germinate for 3 days prior to inoculation.- Disinfect wheat or barley seeds in sterilizing solution for 5 min and wash 3 times with sterile deionized water.
- Imbibe disinfected seeds in 9 cm Petri dishes containing one 8 cm filter paper soaked with sterile deionized water at 4 °C for 1 day. Keep in dark by wrapping plates in aluminium foil.
- Disinfect wheat or barley seeds in sterilizing solution for 5 min and wash 3 times with sterile deionized water.
- Day 4 (wheat) or 5 (barley)
Transfer plates to plant growth chamber but maintain in darkness (wrapped in foil). - Day 6
- Place three 12.5 cm diameter filter paper layers into 14 cm Petri dishes.
- Add sterile deionized water to filter paper until completely soaked.
- Remove excess water from plates.
- Place 16 pre-germinated wheat or barley seeds on filter paper in rows of 3-5-5-3 seeds.
- Seal plates with film and place plates to plant growth chamber in dark overnight.
- Place three 12.5 cm diameter filter paper layers into 14 cm Petri dishes.
- Day 7
- Make mycelial plugs of approximately 5-6 mm diameter by using the reverse of a pipette tip or a cork borer from the edge of the growing colony (Figure 1).
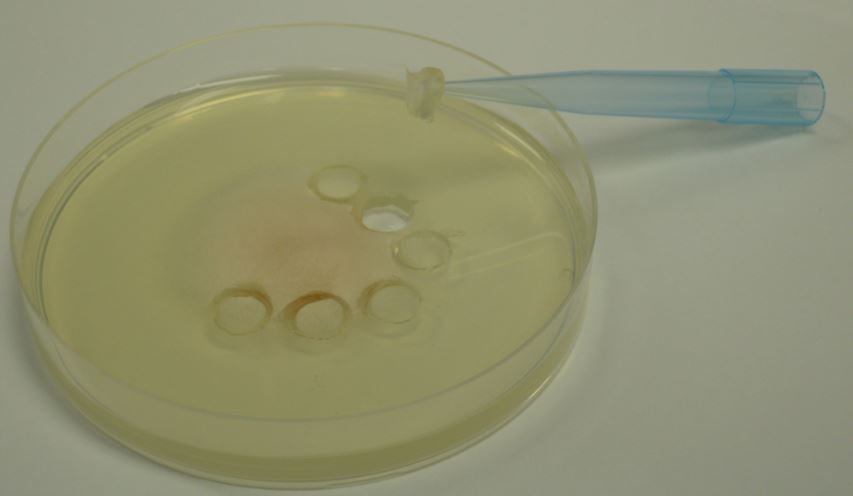
Figure 1. Mycelial plugs obtained from the edge of a growing Fusarium colony.
- Place mycelial plugs upside down on the main root of each germinated seed when about 3 cm long, at approximately 1 cm from the seed, with the mycelium in direct contact with the root (Figure 2).

Figure 2. Mycelial plugs on a wheat rootle. - Seal plates with sealing film or Parafilm.
- Put plates in incubator at 21 ± 1 °C for 4 to 6 days with 12 h of light.
- Make mycelial plugs of approximately 5-6 mm diameter by using the reverse of a pipette tip or a cork borer from the edge of the growing colony (Figure 1).
- Day 11-13 as required
- Determination of root-rot: observation of root necrosis at 4 days post-inoculation by measuring symptom extension (SE) (cm) and visually observing the browning index (BI, 0 = symptomless; 1 = slightly necrotic; 2 moderately necrotic; 3 = severely necrotic; 4 = completely necrotic).
- Determination of crown-rot: Visual observation of crown necrosis at 6 days post-inoculation by a 0-4 scale (0 = symptomless; 1 = slightly necrotic; 2 moderately necrotic; 3 = severely necrotic; 4 = completely necrotic), measuring of shoot length with a ruler (cm) and weighing the shoots after their dissection with a razor blade.
Please see Figure 1 of Beccari et al. (2011) and Figures 6 and 8 of Gardiner et al. (2012).
- Determination of root-rot: observation of root necrosis at 4 days post-inoculation by measuring symptom extension (SE) (cm) and visually observing the browning index (BI, 0 = symptomless; 1 = slightly necrotic; 2 moderately necrotic; 3 = severely necrotic; 4 = completely necrotic).
Acknowledgments
The present protocol here described in detail was adopted in experiments reported in the following publications: Beccari et al. (2011) and Gardiner et al. (2012). Work was supported by the Australian Grains Research and Development Corporation, an Australian statutory authority.
References
- Beccari, G., Covarelli, L. and Nicholson, P. (2011). Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol 60(4): 671-684.
- Gardiner, D. M., McDonald, M. C., Covarelli, L., Solomon, P. S., Rusu, A. G., Marshall, M., Kazan, K., Chakraborty, S., McDonald, B. A. and Manners, J. M. (2012). Comparative pathogenomics reveals horizontally acquired novel virulence genes in fungi infecting cereal hosts. PLoS Pathog 8(9): e1002952.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Covarelli, L., Gardiner, D., Beccari, G. and Nicholson, P. (2013). Fusarium Virulence Assay on Wheat and Barley Seedlings. Bio-protocol 3(7): e446. DOI: 10.21769/BioProtoc.446.
Category
Microbiology > Microbe-host interactions > In vivo model > Plant
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link