- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Flow Cytometric Detection of Mitochondrial Membrane Potential
Published: Vol 3, Iss 8, Apr 20, 2013 DOI: 10.21769/BioProtoc.430 Views: 29939
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
Melissa Dolan [...] John M.L. Ebos
Aug 20, 2025 3878 Views

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1417 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1448 Views
Abstract
Mitochondrial membrane potential (Δψm) is an important parameter of mitochondrial function and an indicator of cell health. Depletion of Δψm suggests the loss of mitochondrial membrane integrity reflecting the initiation of the proapoptotic signal. Recently, lipophilic cationic fluorescent dyes have been developed to detect Δψm by accumulating in the mitochondrial matrix until the Nernstian equilibrium distribution of lipophilic cations is reached. In this protocol, we applied a cell-permeant, green-fluorescent, lipophilic dye 3,3'-dihexyloxacarbocyanine Iodide (DiOC6(3)) which accumulates in mitochondria due to their large negative membrane potential, it can be applied to monitor the mitochondrial membrane potential using flow cytometric detection.
Materials and Reagents
- Cells to analyze (this protocol has been successfully performed on A549, CL1-0, IMR-90, and MCF7 cells)
- Dulbecco's Phosphate-Buffered Saline (DPBS)
- DiOC6(3) (Life Technologies, Molecular Probes®, catalog number: D-273 )
- DMSO (Sigma-Aldrich, catalog number: D8418 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: P6148 )
- 5 ml polystyrene BD falcon round-bottom tube with cell strainer cap (BD Biosciences, catalog number: 352235 )
- Sodium Chloride (NaCl)
- NaOH
- Potassium Chloride (KCl)
- Potassium Phosphate, monobasic (KH2PO4)
- Sodium Phosphate, dibasic (Na2HPO4)
- DPBS (see Recipes)
- DiOC6 stock (see Recipes)
- Paraformaldehyde stock (8%) (see Recipes)
Equipment
- Flow cytometry
- Water bath with temperature control
- Centrifuge
- 6-cm dish
- 0.22 μm filter
Procedure
- Cells were cultured with complete medium in a 6 cm dish at 37 °C and 5% CO2.
- Cells were harvested until 70-90% confluency reached.
- (For positive control of depletion of mitochondria membrane potential) Suspend 1 x 106 cells in 1 ml freshly prepared 4% paraformaldehyde diluted from stock using DPBS for 10 min at room temperature and wash them with 1 ml pre-warmed (37 °C) DPBS three times.
- Dilute the DiOC6 stock solutions into DPBS to make 0.1 μM working solution.
- Suspend cells at a density of 1 x 106 cells/ml in dye working solution and protect from light.
- Incubate the cells at 37 °C for 15 min.
- Centrifuge the tubes at 130 x g for 5 min.
- Remove the supernatant and gently resuspend the cells in 1 ml pre-warmed DPBS.
- Repeat the wash steps 7 and 8 twice.
- Submit samples to flow cytometry for mitochondrial membrane potential measurement.
Data analysis
- Gate on the main cell population.
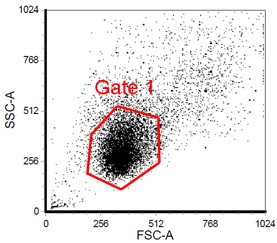
Figure 1. Cells were analyzed according to their size and granularity. The X-axis represents the forward scatter (FSC) parameter which is relative to the size for the cell. The Y-axis shows the side scatter (SSC) parameter which correlates with the components inside the cell. Gate 1 indicates the main population of the cells we analyzed.
- Show the intensity of DiOC6(3) of cells in gate 1.
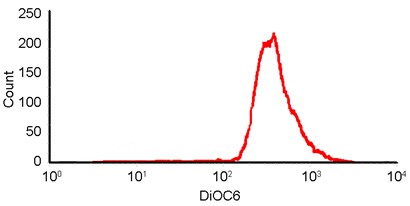
Figure 2. Histogram of DiOC6(3). It shows how many cells are at each intensity of DiOC6(3). The X-axis represents the DiOC6(3) intensity, while the Y-axis indicates the cell counts in corresponding fluorescence intensity.
- Overlap the DiOC6(3) signals of positive control which indicating the population of depletion of mitochondria membrane potential (MMP).
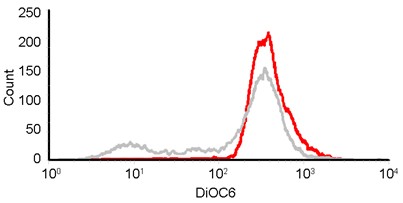
Figure 3. Overlapped histograms of healthy cells (red) and cells loss of mitochondria membrane potential (gray). DiOC6(3) histograms of healthy cells and cells loss of MMP were overlapped to compare the intensity differences.
- Gate on cells loss of MMP (R1) and healthy cells (R2).
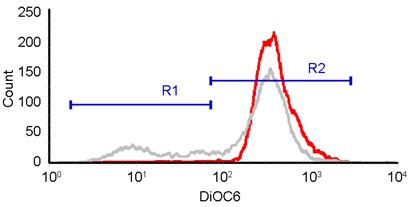
Figure 4. Selection of cell populations in health (R2) or depletion of MMP (R1). Healthy cells with high intensity in DiOC6(3) were gated in R1, while cells loss of MMP with lower DiOC6(3) intensity were gated in R1.
Recipes
- DPBS (1 L)
8 g Sodium Chloride (NaCl)
0.2 g Potassium Chloride (KCl)
0.2 g Potassium Phosphate, monobasic (KH2PO4)
1.15 g Sodium Phosphate, dibasic (Na2HPO4)
Adjust to pH = 7.3. - DiOC6 stock (10 mM)
Dissolve 10 mg in 1.747 ml DMSO to make 1 mM stock.
Aliquot and store at -20 °C.
Avoid from light and repeated freeze/thaw cycles. - Paraformaldehyde stock (8%)
Weight 8 g paraformaldehyde to 90 ml distilled water (in a fume hood).
Add 0.1 ml of 10 N NaOH.
Heat and stir the solution until the granules are fully dissolved (do not heat the solution above 65 °C).
Turn off the heater and adjust to pH 7.4 with about 0.3 ml of 20% HCl.
Bring volume to 100 ml with distilled water.
Sterilize the solution with 0.22 μm filter and can be stored at 4 °C for 30-60 days.
References
- Chang, H. Y., Huang, H. C., Huang, T. C., Yang, P. C., Wang, Y. C. and Juan, H. F. (2012). Ectopic ATP synthase blockade suppresses lung adenocarcinoma growth by activating the unfolded protein response. Cancer Res 72(18): 4696-4706.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chang, H., Huang, H., Huang, T., Yang, P., Wang, Y. and Juan, H. (2013). Flow Cytometric Detection of Mitochondrial Membrane Potential. Bio-protocol 3(8): e430. DOI: 10.21769/BioProtoc.430.
Category
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











