- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Plasmodium falciparum Rosette Disruption Assay
Published: Vol 3, Iss 8, Apr 20, 2013 DOI: 10.21769/BioProtoc.411 Views: 10997

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Intracellular Invasion and Killing Assay to Investigate the Effects of Binge Alcohol Toxicity in Murine Alveolar Macrophages
Victor Jimenez Jr and Fernando P Monroy
Jan 20, 2019 6743 Views

Model of Chemotherapy-associated Mucositis and Oral Opportunistic Infections
Takanori Sobue [...] Anna Dongari-Bagtzoglou
Nov 5, 2019 5326 Views

Differential Fractionation of Erythrocytes Infected by Plasmodium berghei
Bénédicte Gnangnon [...] Christine Pierrot
Jun 5, 2020 4476 Views
Abstract
Rosetting, i.e. the capacity of Plasmodium falciparum-infected red blood cells (iRBCs) to bind two or more uninfected red blood cells (RBCs) is associated with severe malaria in African children. Disruption of rosettes using small soluble inhibitors or specific antibodies is viewed as an interesting strategy to treat or prevent severe malaria manifestations. The protocol presented here describes an assay to monitor rosette dissociation, validated for the Palo Alto VarO, IT4/R29 and 3D7/PF13 rosetting clones (Vigan-Womas et al., 2011).
Materials and Reagents
- Rosette-forming iRBCs
- Culture of rosette-forming parasites at mature stages (trophozoite to early schizont stages, 5-10% parasitaemia, 5% haematocrit)
- Parasite clonal lines 3D7/PF13, Palo Alto 89F5 VarO, IT4/R29 or patient isolates
- Culture of rosette-forming parasites at mature stages (trophozoite to early schizont stages, 5-10% parasitaemia, 5% haematocrit)
- Culture medium reagents
- Malaria non-immune human AB+ serum (pool of 3-5 different donors)
- RPMI 1640 medium with L-glutamine and 25 mM HEPES (500 ml) (Life Technologies, Gibco®, catalog number: 52400 )
- 10 mM Hypoxanthine solution (100x) (C.C.Pro, catalog number: Z-41-M )
- Gentamicin solution (50 mg/ml) (Sigma-Aldrich, catalog number: G1397 )
- Malaria non-immune human AB+ serum (pool of 3-5 different donors)
- Other materials
- Non pyrogenic sterile polystyrene, Rectangular Canted Neck Cell Culture Flask with Plug Seal Cap (75 cm2, Corning Incorporated, catalog number: 430720 ; 25 cm2, Corning Incorporated, catalog number: 430168 , for 20 and 5 ml culture medium, respectively)
- Ice cold Ficoll (LymphoprepTM, density 1.077 g/ml) (Abcys, catalog number: 1114545 )
- Dextran sulfate sodium salt from Leuconostoc spp, MW > 500,000 (Sigma-Aldrich, catalog number: D6001 )
- Heparin sodium salt from porcine intestinal mucosa (Sigma-Aldrich, catalog number: H3393 )
- Fucoidan from Fucus vesiculosus (Sigma-Aldrich, catalog number: F5631 )
- Hoechst 33342, 10 mg/ml solution in water (Life Technologies, Molecular Probes®, catalog number: H-3570 )
- Sterile distilled water RNase DNase free (Life Technologies, Gibco®, catalog number: 10977-035 )
- Mouse, rat or rabbit immune and pre-immune polyclonal sera
- Monoclonal antibodies (mAbs) reacting with the surface of rosette-forming iRBCs
- Serum or plasma samples from individuals living in a malaria endemic region
- Serum or plasma samples from malaria non-immune individuals
Note: All plasma samples must be collected using an anticoagulant other than heparin (heparin disrupts rosettes of multiple strains at the concentration used for anticoagulant activity. Convenient anticoagulants are EDTA [ethylene diamine tetraacetic acid] or sodium citrate). Store at -20 °C or -80 °C until use.
Note: Abs are home made or collected from patients.
- Non pyrogenic sterile polystyrene, Rectangular Canted Neck Cell Culture Flask with Plug Seal Cap (75 cm2, Corning Incorporated, catalog number: 430720 ; 25 cm2, Corning Incorporated, catalog number: 430168 , for 20 and 5 ml culture medium, respectively)
Equipment
- 5 ml polystyrene round-bottom sterile tube (BD Biosciences, Falcon®, catalog number: 352058 )
- Sterile laboratory vacuum filter 0.22 µm (Stericup® Filter Units Millipore, catalog number: 051446 )
- Sterile polypropylene conical centrifuge tubes (15 ml, BD Biosciences, Falcon®, catalog number: 352097 ; 50 ml, BD Biosciences, Falcon®, catalog number: 352098 )
- Sterile disposable serological 1 ml aspiration pipette (Dominique Dutscher, catalog number: 999079 )
- Professional gloves (e.g. Kimtech Sterling Nitrile gloves, Kimberley-Clark Professional, catalog number: 99211 , or Satin Plus gloves Kimberley-Clark Professional, catalog number: SP2330E or 2220E )
- 1.4 ml matrix round bottom non sterile tubes (Thermo Fisher Scientific, catalog number: 10630784 )
- Microscope glass slides (Thermo Fisher Scientific, catalog number: 10090431 ) and cover slips 22 x 22 mm (Thermo Fisher Scientific, catalog number: 11728691 )
- Centrifuge with a swing bucket rotor (Thermo Fisher Scientific Heraeus Multifuge 3SR+ centrifuge )
- Centrifuge Eppendorf 5702 with an A-4-38 swing bucket rotor (Thermo Fisher Scientific, catalog number: 05-400-318)
- Vacuum pump (ILMVAC Biovac, model: 104 )
- Fluorescence microscope with UV-light and 40x or 100x magnification (Leica fluorescence microscope DM5000B, HP Plan ocular 10 x 22 507897, HCX Plan 100x Oil, HCXPL 40x PH2)
- Incubator at 37 °C in continuous gazing: 5% O2, 5% CO2 and 90% N2 (Thermo Fisher Scientific Binder incubator, model: CB210 )
- Laminar flow class II, type A2 biological safety cabinet (e.g. Thermo Fisher Scientific HeraSafe, catalog number: 13-998-002 )
Procedure
Please note that gloves must be used throughout the handling procedures.
- Preparation of rosettes
- Prepare parasite cultures of rosette-forming parasites (see "Plasmodium falciparum Rosette formation assay", sections 1 and 2). Prepare enough material to perform each assay in triplicate: Each assay requires 3 x 40 μl rosette-rich suspension at 2% haematocrit (see 1.i-j.). This corresponds to an initial 20 μl culture at 6-8% parasitemia i.e. approx.
- Harvest the cultures of rosette-forming parasites at mature trophozoite to schizont stages (5- 10% parasitaemia). Avoid late or segmented schizonts, which form loose rosettes.
- Enrich for rosettes by centrifugation on ice-cold Ficoll (see "Plasmodium falciparum Rosette formation assay"- section 2). Rosette disruption assays are best carried out with parasite cultures with more than 50% rosette frequency.
- Spin down the cells by centrifugation in the Fisher Scientific Heraeus Multifuge for 10 min at 256 x g (1,200 rpm), room temperature.
- Discard the supernatant by aspiration using a 1 ml aspiration sterile pipette connected to a vacuum pump.
- Gently resuspend the rosette-rich pellet (some rosettes tend to be disrupted by simple mechanical handling, avoid back and forth harsh pipetting) in 4 ml pre-warmed CCM (see Recipes) supplemented with 10 μg/ml Hoechst.
- Mix gently and incubate for 10 min at 37 °C in the dark in an incubator with continuous gazing (5% O2, 5% CO2, 90% N2).
- Wash once with CCM: Add 900 μl CCM, centrifuge 3 min at 256 x g (1,200 rpm), in the Fisher Scientific Heraeus Multifuge at room temperature.
- Remove the supernatant and resuspend the pellet at 2% haematocrit in CCM (2 volumes (vol.) cell pellet + 98 vol. CCM). Parasitaemia of the preparation is usually around 5%.
- Dispense 40 μl aliquots of the rosette-rich RBC suspension in 1.4 matrix round bottom tubes.
- Centrifuge, in the Fisher Scientific Heraeus Multifuge 5 min at 178 x g (1,000 rpm) room temperature.
- Discard the supernatant and process the pellet as described in section 2 or 3.
- Prepare serial dilutions (0.1 μg/ml - 1 mg/ml) of heparin, dextran sulfate, fucoidan or other sulphated glycans in CCM.
- Prepare serial dilutions of sera, plasma (starting dilution 1/5) or purified mAbs or immunoglobulin preparations in CCM as diluent. Include negative controls such as CCM alone and non-immune serum (or Ig) and a positive control with sera or mAbs able to disrupt rosettes.
- Prepare parasite cultures of rosette-forming parasites (see "Plasmodium falciparum Rosette formation assay", sections 1 and 2). Prepare enough material to perform each assay in triplicate: Each assay requires 3 x 40 μl rosette-rich suspension at 2% haematocrit (see 1.i-j.). This corresponds to an initial 20 μl culture at 6-8% parasitemia i.e. approx.
- Dissociation assay with sulphated glycans
- Add 100 μl diluted sulphated glycan to the rosette preparation (section 1-j).
- Incubate for 30 min at 37 °C (use an incubator rather than water-bath).
- Remove 60 μl of medium and resuspend the sedimented pellet in the remaining volume.
- Take a 10 μl aliquot, put it onto a microscope slide and add a cover slip.
- Count under the microscope 100-200 iRBCs and score iRBCs engaged in rosettes (i.e. trophozoite or schizont stages having bound two or more uninfected RBCs) (Figure 1).
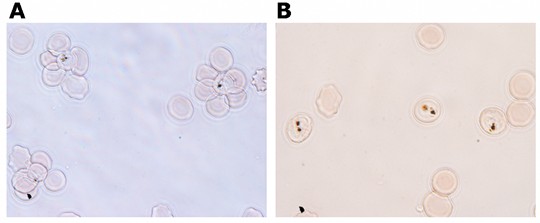
Figure 1. Rosette dissociation assay before A and after B incubation with 10 μg/ml dextran sulphate. - Calculate the rosetting rate = (No. mature stage iRBCs engaged in rosettes/No. mature stages) x 100.
- Calculate the dissociation rate = (rosetting rate of control culture without inhibitor - rosetting rate of treated sample/rosetting rate of control culture without inhibitor) x 100.
- Add 100 μl diluted sulphated glycan to the rosette preparation (section 1-j).
- Dissociation assay with immune reagents
- Prepare serial dilutions of sera, plasma (starting dilution 1/5) or purified mAbs or immunoglobulin preparations to be tested. Include negative controls such as CCM alone and non-immune serum (or Ig) and a positive control with sera or mAbs able to disrupt rosettes.
- Add 40 μl diluted serum or plasma to the rosette preparation (section 1-n).
- Incubate for 30 min at 37 °C (use an incubator rather than water-bath).
- Resuspend the sedimented pellet in the incubation medium.
- Take a 10 μl aliquot, put it onto a microscope slide and add a cover slip.
- Count under the microscope, 100-200 iRBCs and score iRBCs engaged in rosettes.
- Calculate the rosetting rate = (No. mature stage iRBCs engaged in rosettes/No. mature stages) x 100.
- Calculate the dissociation rate = (rosetting rate of control culture incubated with non immune reagent - rosetting rate of treated sample/rosetting rate of control culture incubated with non immune reagent) x 100.
Note that at certain dilutions, polyclonal immune reagents (and IgM mAbs) tend to disrupt rosettes and agglutinate iRBCs. Immune agglutinates are easily visualised as densely packed iRBCs (see Figure 2). Dissociation is readily visualised at higher dilutions.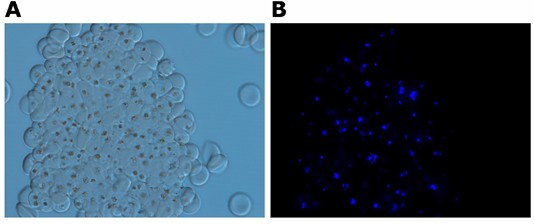
Figure 2. Agglutination of rosette-forming iRBCs obtained after incubation with mouse polyclonal antibodies directed against a PfEMP1-derived surface exposed domain (dilution 1/40). The iRBC nuclei were stained with Hoechst 33342 and cells were examined by light A and UV B microscopy.
- Prepare serial dilutions of sera, plasma (starting dilution 1/5) or purified mAbs or immunoglobulin preparations to be tested. Include negative controls such as CCM alone and non-immune serum (or Ig) and a positive control with sera or mAbs able to disrupt rosettes.
Recipes
- Complete culture medium (CCM, 500 ml)
445 ml RPMI 1640
5 ml Hypoxanthine solution (final concentration, 100 μM)
200 μl Gentamicin solution (final concentration 20 μg/ml)
50 ml Human AB+ serum (final concentration 10%)
Sterilize using a 0.22 μm filter unit
Store at 4 °C or -20 °C for long-term storage - Stock solutions of sulphated glycans
Prepare a 10 mg/ml solution of sulphated glycan in sterile water (0.1 g + 10 ml water)
Sterilize using a 0.22 μm filter unit
Aliquot and store at -80 °C
Use each aliquot once (do not freeze-thaw)
Acknowledgments
This protocol was adapted from the following publications: Vigan-Womas et al. (2008 and 2010). This work was supported by the Agence Nationale de la Recherche, contract ANR-07-MIME-021-0 (www.agence-nationale-recherche.fr/), and the 7th European Framework Program, FP7/2007-2013, (http://cordis.europa.eu/fp7/home_en.html) contract 242095, Evimalar.
References
- Vigan-Womas, I., Guillotte, M., Le Scanf, C., Igonet, S., Petres, S., Juillerat, A., Badaut, C., Nato, F., Schneider, A., Lavergne, A., Contamin, H., Tall, A., Baril, L., Bentley, G. A. and Mercereau-Puijalon, O. (2008). An in vivo and in vitro model of Plasmodium falciparum rosetting and autoagglutination mediated by varO, a group A var gene encoding a frequent serotype. Infect Immun 76(12): 5565-5580.
- Vigan-Womas, I., Lokossou, A., Guillotte, M., Juillerat, A., Bentley, G., Garcia, A., Mercereau-Puijalon, O. and Migot-Nabias, F. (2010). The humoral response to Plasmodium falciparum VarO rosetting variant and its association with protection against malaria in Beninese children. Malar J 9: 267.
- Vigan-Womas, I., Guillotte, M., Juillerat, A., Vallieres, C., Lewit-Bentley, A., Tall, A., Baril, L., Bentley, G. A. and Mercereau-Puijalon, O. (2011). Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes. PLoS One 6(1): e16544.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Guillotte-Blisnick, M., Mercereau-Puijalon, O. and Vigan-Womas, I. (2013). Plasmodium falciparum Rosette Disruption Assay. Bio-protocol 3(8): e411. DOI: 10.21769/BioProtoc.411.
Category
Microbiology > Microbe-host interactions > In vitro model > Cell line
Cell Biology > Cell staining > Nucleic acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link