- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Lung Clearance Assay
Published: Vol 3, Iss 5, Mar 5, 2013 DOI: 10.21769/BioProtoc.336 Views: 11138
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2529 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3550 Views
Abstract
Lung clearance assay tests the ability of innate immune cells (mainly NK cells) to eradicate in vivo cells injected via the tail vein of the mice. This assay helps to elucidate the role played by NK cells and their receptors (if the mice are genetically modified) against various human and mouse targets in an in vivo setting (Stern-Ginossar et al., 2008; Halfteck et al., 2009; Tsukerman et al., 2012).
Materials and Reagents
- Cell labeling dyes
- Vybrant CM-DiI (Life Technologies, InvitrogenTM, catalog number: V-22888 )
- VybrantDiD (Life Technologies, InvitrogenTM, catalog number: V-22887 )
- CellTrace CFSE (Life Technologies, InvitrogenTM, catalog number: C-34554 ) (either CM-DiI or CFSE can be used based on the preferred wave length either FL-1 for CFSE or FL-2 for the CM-DiI)
- Vybrant CM-DiI (Life Technologies, InvitrogenTM, catalog number: V-22888 )
- Other materials
- Phosphate buffered saline (PBS)
- 1x Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen,Life Technologies, InvitrogenTM, catalog number: 10313-039 )
- Fetal bovine serum (Life Technologies, InvitrogenTM, catalog number: 11091-148 )
- 40 micron cell strainer (BD Biosciences, Falcon®, catalog number: 352340 )
- Mice of interest
- NaN3 (Sodium Azide)
- Erythrocyte lysis buffer (ELB) (see Recipes)
- FACS buffer (see Recipes)
- Phosphate buffered saline (PBS)
- Antibodies
To demonstrate that the observed effect is specific we recommend to either blocking the relevant receptors, or by depleting NK cells, by using anti-NK1.1 (in case the receptor is present), or by using anti-asialo GM1.- Anti NK1.1 (eBioscience, catalog number: 14-5941-82 )
- Anti-Asialo-GM1 (eBioscience, catalog number: 16-6507-39 )
- Anti-Mouse CD314 (NKG2D) (eBioscience, catalog number: 14-5873-82 )
- Anti NK1.1 (eBioscience, catalog number: 14-5941-82 )
Equipment
- BD LSR II flow cytometer
Procedure
- The tail vein injection requires prior practice, mature male mice are easier to inject. We suggest using 8-12 weeks male mice.
- The depletion of NK cells for a period of one day is obtained by I.P (Intra peritoneal) injection of 150 microgram (in up to 250 μl PBS) of anti NK1.1 up to 24 h prior to the experiment.
- Note: Not all cell types are suitable for these experiments, small cells are hard to recover, large cells may clog the lungs, some cells are not labeled well etc. Therefore, preliminary experiments using limited amount of animals are strongly recommended. The lines that we tested are: HeLa, PC-3, DU 145, PD1.6 and YAC-1. Since the tail vein injections may vary between different mice even in the same group there is a need for an internal injection control which is usually cells that are resistant to mouse NK cell killing. Label two cell types (experiment and control) according to the manufacturer instructions for the dyes. If the cells used were not in the manufacturer protocol calibration in vitro staining experiments are required. Labeling of HeLa (the control population for the majority of our experiments), Jurkat, 3T3, P3X, PC-3 DU 145 is performed as follows:
- Suspend cells at a density of 1 x 106/ml in serum-free culture medium (DMEM or RPMI).
- Add 1- 5 μl (from the stock) of the cell-labeling solution per ml of cell suspension. Mix well by gentle pipetting. Do not exceed 5 ml volume in each labeling tube. The differences between CFSE and Vybrant-CM-DiI are, the emission (FL-1/FL-2) and the range of labeled cells (we recommend working with the Vybrant-CM-DiI when possible).
- Incubate at 37 °C HeLa (8 min), Jurkat (2 min), 3T3 (15 min), P3X (15 min), PC-3 (30 min) DU 145 (30 min). We recommend incubating in 15 ml tubes covered with aluminum foil.
- Centrifuge at 450-515 x g for 5 min.
- Remove the supernatant and gently resuspend the cells in warm (37 °C) medium.
- Repeat the wash procedure (3-d and 3-e).
- Suspend cells at a density of 1 x 106/ml in serum-free culture medium (DMEM or RPMI).
- Resuspend the cells to a final concentration of 107 cells per ml these cells are to be injected to the mice, 2 x 106cells of each cell type (4 x 106 total) are injected via the tail vein per mouse; the final injection volume is 400 μl.
- Pass the cells through a 40 μm cell strainer.
- Set aside 400 μl of the cell mixture that will serve as the pre-injection tube. Keep these cells at 37 °C for the duration of the experiment.
- Inject the cells into the tail vein of the mice.
- Wait for 5 h.
- Sacrifice the mice according to ethical regulations, we recommend using CO2.
- Harvest the lungs, place them in a 6 well plates with 2 ml ice cold PBS until harvesting the lungs of the entire experiment is completed.
- Place a 40 μm cell strainer on top of a 50 ml tube, transfer the lungs into the strainer, transfer the PBS from the 6 well into the tube.
- Smash the lungs by using the cylinder/pestle of a 5 ml syringe; add more PBS as required.
- After obtaining a homogenous solution centrifuge the tubes at 450-515 x g for 5 min.
- Discard supernatant and resuspend cells with 10 ml of ELB (erythrocyte lysis buffer) and keep on ice for 5 min.
- Centrifuge the cells at 450-515 x g for 5 min.
- Discard supernatant and observe for erythrocyte content (if there is visible amount of red cells in the pellet) repeat steps 15-16, until the red blood cells disappear.
- Resuspend the cells in 10 ml of DMEM/RPMI supplemented with 10% FBS (this step eliminates the ELB activity).
- Centrifuge the cells at 450-515 x g for 5 min.
- Discard supernatant and resuspend cells in 1-2 ml of FACS buffer according topellet size.
- Examine the cells using FACS. Begin with the pre-injection sample, set the gates based on forward and side scatter (see figure below), and calibrate the laser intensity. Collect at least 2.5 x 105 cells. Draw a dot plot of FL-4 vs FL-2 (if using DiI) or FL-4 vs FL-1 (if using CFSE).
- Read the lung samples. We recommend at collecting least 2.5 x 106 cells per sample
- Note: cells can be resuspended in 2% formaldehyde and stored overnight at 4 °C in dark, prior to analysis by flow cytometry.
- Flow cytometric analysis (Figure 1): Y axis FL-1 intensity X axis FL-4 intensity. The square frame marks the “experiment” cell population; the dashed square frame marks the “internal control” cell population. The lower left square are lung cells of the mice ( FL-1, FL-4 negative).
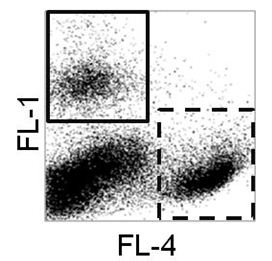
Figure 1. Flow cytometric analysis - To obtain the killing percentage, the amount of experimental cells (NK sensitive) divided by the amount of the control cells (NK insensitive) in the pre-injection group is calculated and this ratio is set up to be the reference control. Accordingly, similar calculation is performed regarding the cells recovered in the lungs 5 h post infection and tumor cell survival is calculated.
Recipes
- ELB (erythrocyte lysis buffer)
NH4Cl 16.4 g
KHCO3 2 g
EDTA 0.5 M 400 μl
2 L ddH2O
Titrate with HCl to pH 7.2-7.4 - FACS buffer
1x PBS
0.5% BSA
0.05% NaN3 (Sodium Azide)
Acknowledgments
The authors would like to acknowledge Noam Stern-Ginossar, Chamutal Gur, Ariella Glasner, and Mazal El-Nekaveh.
References
- Halfteck, G. G., Elboim, M., Gur, C., Achdout, H., Ghadially, H. and Mandelboim, O. (2009). Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol 182(4): 2221-2230.
- Stern-Ginossar, N., Gur, C., Biton, M., Horwitz, E., Elboim, M., Stanietsky, N., Mandelboim, M. and Mandelboim, O. (2008). Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 9(9): 1065-1073.
- Tsukerman, P., Stern-Ginossar, N., Gur, C., Glasner, A., Nachmani, D., Bauman, Y., Yamin, R., Vitenshtein, A., Stanietsky, N., Bar-Mag, T., Lankry, D. and Mandelboim, O. (2012). MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res 72(21): 5463-5472.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tsukerman, P. and Mandelboim, O. (2013). Lung Clearance Assay. Bio-protocol 3(5): e336. DOI: 10.21769/BioProtoc.336.
Category
Cancer Biology > Tumor immunology > Animal models > Cell viability
Immunology > Immune cell function > Lymphocyte
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











