- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Cochlear Whole Mount Immunofluorescence
Published: Vol 3, Iss 5, Mar 5, 2013 DOI: 10.21769/BioProtoc.332 Views: 33287

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1830 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3773 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2636 Views
Abstract
This protocol comprises the entire process of immunofluorescence staining on mouse cochlea whole mount, starting from tissue preparation to the mounting of the tissue. This technique provides “three-dimensional” views of the stained components in order to determine the localization of a protein of interest in the tissue in its natural state and environment.
Keywords: Inner earMaterials and Reagents
- Ketamine hydrochloride (100 mg/ml) (Ketaset FORT DODGE)
- Xylazine hydrochloride (100 mg/ml) (Xylazine AnaSed)
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: P6148 )
- Sodium Phosphate monohydrate (NaH2PO4.1H2O) (Sigma-Aldrich, catalog number: S9638 )
- Sodium Phosphate heptahydrate (NaH2PO4.7H2O) (Sigma-Aldrich, catalog number: S9390 )
- Sodium Hydroxide (NaOH) (Thermo Fisher Scientific, catalog number: BP359 )
- Sodium Chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Phosphate buffer (PB)
- Phosphate buffer saline (PBS)
- EDTA (Sigma-Aldrich, catalog number: E7889 )
- Blocking buffer
- Normal goat serum (NGS) (Sigma-Aldrich, catalog number: G-6767 )
- Saponin (Sigma-Aldrich, catalog number: 47036 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Primary Antibodies
- Rabbit anti-myosin VIIa antibody (a hair cell-specific marker) (Proteus Biosciences, catalog number: 25-6790 ) at a dilution of 1:50 in blocking buffer.
- Guinea pig anti- VGLUT3 (Vesicle Glutamate Transporter 3) antibody at 1:5,000 in blocking buffer (a gift from Dr. Robert Edward, Department of Neurology, UCSF)
- Rabbit anti-myosin VIIa antibody (a hair cell-specific marker) (Proteus Biosciences, catalog number: 25-6790 ) at a dilution of 1:50 in blocking buffer.
- Secondary Antibodies
- Cy2-conjugated Goat anti-rabbit secondary IgG antibody (Jackson ImmunoResearch) at a dilution of 1:4,000 in PBS.
- Cy3-conjugated Goat anti-guinea pig secondary IgG antibody (Jackson ImmunoResearch) at a dilution of 1:4,000 in PBS.
Note: secondary antibodies should be diluted in PBS (not blocking buffer).
- Cy2-conjugated Goat anti-rabbit secondary IgG antibody (Jackson ImmunoResearch) at a dilution of 1:4,000 in PBS.
- DAPI to counterstain nuclei (1.5 μg/ml in PBS) (Sigma-Aldrich, catalog number: D9564 ).
- Antifade FuorSave reagent (Calbiochem, catalog number: 34589 )
- 10x PB (see Recipes)
- 10x PBS (see Recipes)
- 4% PFA-PB (50 ml) (see Recipes)
- Blocking buffer (see Recipes)
Equipment
- Syringe (1 ml syringe 25G1) (BD Bioscience, catalog number: 329622 )
- 0.5 ml microcentrifuge tube’s caps (Thermo Fisher Scientific, catalog number: 02-681-311 )
- Microdissecting scissor (Fine Science Tools, catalog number: 15002-08 )
- Fine forceps (Ted Pella, catalog number: 5621 )
- Surgical blade (Feather)
- 5 ml Vials (Denville, catalog number: T8200-S )
- Pasteur pipettes (used to rinse the tissue) (Thermo Fisher Scientific, catalog number: 13-678-20 )
- SuperFrost slides (Thermo Fisher Scientific, catalog number: 12-550 )
- Cover slips (Thermo Fisher Scientific, catalog number: 12-548-5A )
- Petri dish (BD Biosciences, Falcon®, catalog number: 1007 )
- Aluminum foil
- Refrigerator
- Rocking platform
- Dissecting microscope
- Confocal microscope
Procedure
- Cochlea extraction and perfusion
- Anesthetize mice (mice overdosed with 200 μl of a mixture of ketamine hydrochloride, 100 mg/ml and xylazine hydrochloride 10 mg/ml, per 100 g of mouse weight).
- Pinch the toes to check if the animal is fully asleep then decapitate the mouse.
- Quickly and carefully remove cochleae from skull and put them in a Petri dish as follow:
- Cut the mouse head in half.
- Remove brain.
- Hold head on side so the 'bowl' of the skull is facing up.
- The temporal bone is in the back and is not continuous to the skull (there are many small bones attaching it to the skull).
- Push in the junctions between the skull and the temporal bone (away from the cochlea area) to break it away from skull.
- Carefully dissect extraneous bones away from the cochlea.
The following video by Parker et al. (2010) shows, between min 3 and 4, how to dissect the temporal bone (cochlea) from a P3-5 mouse. The same procedure is done for an older mouse, but the only difference is the bone cochlea becomes harder.
http://www.jove.com/video/1685/primary-culture-plasmid-electroporation-murine-organ
- Cut the mouse head in half.
- Use a syringe to push approximately 0.5 ml of ice cold 4% PFA-PB through the round and oval windows (RW and OW) after having made a small hole in the apex of the cochlea (for orientation refer to the whole cochlea picture with legends in Figure 1 (Glen et al., 2008)).
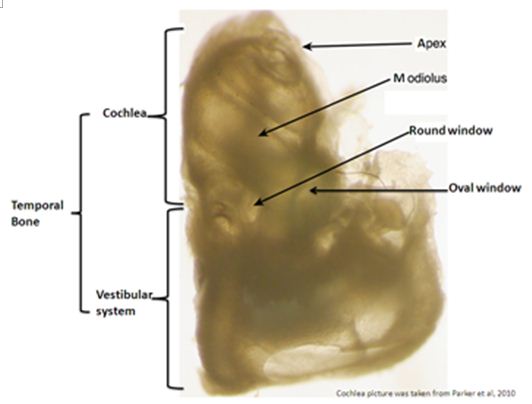
Figure 1. Shows a mouse decalcified left temporal bone. This labeled picture helps for orientation on the mouse anatomy of the whole temporal bone needed for good success in reproducing this protocol [this picture was borrowed from MacDonald et al. (2008) paper].
Note: To have a very good fixation, the round window membrane should be punctured and the stapes should be removed before cochlea perfusion. - Post-fix the cochleae in 5 ml 4% PFA-PB for 2 h at 4 °C under gentle agitation.
- Using 1 ml syringe rinse cochlea by perfusing 3 times PB through the RW and OW.
- Wash 3 x 15 min in fresh PB at 4 °C under gentle agitation.
- Anesthetize mice (mice overdosed with 200 μl of a mixture of ketamine hydrochloride, 100 mg/ml and xylazine hydrochloride 10 mg/ml, per 100 g of mouse weight).
- Cochlea decalcification and organ of Corti dissection into surface preparation
- Transfer the cochleae to 5 ml tube containing 5% EDTA and incubate at 4 °C under gentle agitation.
Post-natal Age < P7 P7-P15 P15-P21 P21 Time in 5% EDTA 1-2 h 2-4 h 5-8 h 12- 24 h
Note: Before going to next step, you need to check if the cochleae are very well decalcified. Insufficient decalcification will not help to get an intact and good organ of Corti. A good decalcification is when the cochleae become soft at touch with forceps and when the cochlea bone becomes transparent. A picture of decalcified cochlea is present in the following video.
http://www.masseyeandear.org/research/ent/eaton-peabody/epl-histology-resources/video-tutorial-for-cochlear-dissection/ - Wash 3 x 15 min in fresh PB at 4 °C under gentle agitation.
- The otic capsule, the lateral wall, Reissner’s membrane and tectorial membrane should be removed in that order and then dissect the organ of Corti (for orientation on the cochlea anatomy see the cochlea mid-turn cross section Figure 2).

Figure 2. Shows a mid-modular cross section of the mouse cochlea turn stained with toluidine blue. This labeled 20x view section helps for orientation on the mouse cochlea anatomy needed in this protocol for good success in reproducing this protocol. - The remaining organ of Corti was further dissected into a surface preparation and microdissected into individual turns.
Note: The video of Liberman's laboratory shows a method of surface preparation
dissection that better preserves the overall structure of the organ of Corti.
http://www.masseyeandear.org/research/ent/eaton-peabody/epl-histology-resources/video-tutorial-for-cochlear-dissection/
- Transfer the cochleae to 5 ml tube containing 5% EDTA and incubate at 4 °C under gentle agitation.
- Immunofluorescence
- Microdissected cochlea individual turns were then placed in a 0.5 ml Ependorf tube cap containing blocking buffer and incubated for 1 h at room temperature (RT) on a rocking platform.
- Incubate in primary antibodies diluted in blocking buffer overnight at 4 °C on a rocking platform.
Note: Putting the cap with cochlea turns in a small Petri dish and wrapping them with parafilm will prevent dehydration. - Rinse 3 x 15 min in PBS.
- Incubate with secondary antibodies in PBS for 2 h at RT on a rocking platform.
Note: Because the secondary antibodies are sensitive to light, the tissue should be wrapped with aluminum foil to protect it from light. - Rinse 3x 15 min in PBS.
- Stain the nuclei with DAPI in PBS for 15 min at RT.
- Rinse 3 x 15 min in PBS.
- Mount the cochlea whole mount turn carefully onto slides.
Note: The orientation is very important in mounting the whole mount organ of Corti. The hair cells stereocilia should face up and be in contact with the coverslip, while the basilar membrane labeled green in Figure 2 should be on the bottom and be in contact with the slide. The video of Liberman's laboratory shows the correct orientation of the cochlea surface preparation a slide of the video is presented in Figure 3.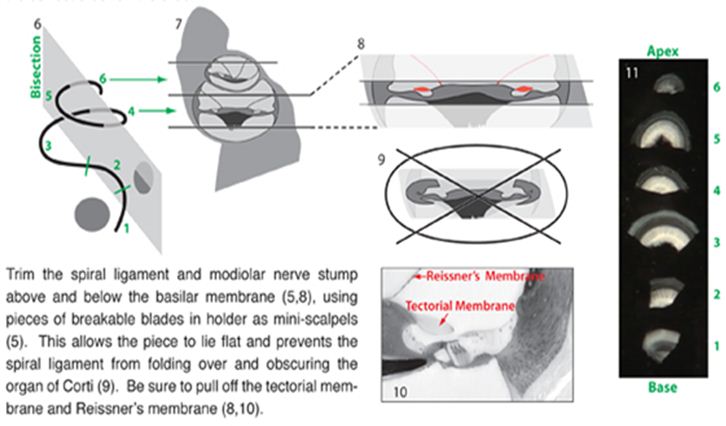
Figure 3. Shows a slide of the video of Liberman's laboratory. “http://www.masseyeandear.org/research/ent/eaton-peabody/epl-histology-resources/video-tutorial-for-cochlear-dissection/” presenting the correct orientation and preparation of the whole mount organ of Corti. The hair cells stereocilia should face up and be in contact with the coverslip, while the basilar membrane labeled green in Figure 2 should be on the bottom and be in contact with the slide. At this step if the orientation of the cochlea surface preparation is done properly it provides very good views of the stained components under the microscope. - Apply a drop of antifades solution, coverslip and then let it dry.
- Slides are now ready to be observed under a microscope equipped with epifluorescence.
Note: For better images use a confocal immunofluorescence microscope and do not expose your slides too much to light to avoid stain bleaching. - Figure 2 in Akil et al. (2012) Neuron paper is a sample of the Mouse Cochlear Whole Mount Immunofluorescence.
- Microdissected cochlea individual turns were then placed in a 0.5 ml Ependorf tube cap containing blocking buffer and incubated for 1 h at room temperature (RT) on a rocking platform.
Recipes
- 10x PB
Dissolve 0.23 g anhydrous NaH2PO4 and 2.17 g Na2HPO4 heptahydrate into 80 ml ddH2O. Adjust pH to 7.4 and bring the final volume to 100 ml - 10x PBS
Dissolve 0.23 g anhydrous NaH2PO4 and 2.17 g Na2HPO4 heptahydrate, and 8.75 g NaCl into 80 ml ddH2O. Adjust pH to 7.4 and bring the final volume to 100 ml - 4% PFA-PB (50 ml)
Dissolve 2 g PFA in approximately 40 ml in ddH2O
(Note: 1-You will need to heat the water and add NaOH to dissolve the PFA. 2- Keep the temperature below 60 °C during the entire process to avoid breakdown of the PFA polymers)
After the PFA is dissolved, add 5 ml of 10x PB. Cool to RT and adjust the pH to 7.4. Bring the final volume to 100 ml. Filter and store at 4 °C
Note: Use this solution within a week or less - Blocking buffer
20% normal goat serum (NGS) with 0.3% TritonX-100 and 0.3% Saponin in PBS
Acknowledgments
We acknowledge the support of this work by Hearing Research Inc and NIH R21 DC012118-01. We thank Neuron Journal and the Mass Eye and Ear for some illustrating images we used in this paper.
References
- Akil, O., Seal, R. P., Burke, K., Wang, C., Alemi, A., During, M., Edwards, R. H. and Lustig, L. R. (2012). Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75(2): 283-293.
- MacDonald, G. H. and Rubel, E. W. (2008). Three-dimensional imaging of the intact mouse cochlea by fluorescent laser scanning confocal microscopy. Hear Res 243(1-2): 1-10.
- Parker, M., Brugeaud, A., Edge, A. S. B. (2010). Primary culture and plasmid electroporation of the murine organ of corti. J Vis Exp (36) e1685.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Akil, O. and Lustig, L. R. (2013). Mouse Cochlear Whole Mount Immunofluorescence. Bio-protocol 3(5): e332. DOI: 10.21769/BioProtoc.332.
Category
Neuroscience > Development > Immunofluorescence
Developmental Biology > Morphogenesis
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











