- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Airway Responsiveness on Vigil and Unrestrained Mouse
Published: Vol 3, Iss 4, Feb 20, 2013 DOI: 10.21769/BioProtoc.328 Views: 9704

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2530 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2552 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views
Abstract
Airway hyperresponsiveness to methacholine is an important characteristic of asthma. This protocol describes how to measure airway response to methacholine in vigil and unrestraint mouse. The enhanced pause (PenH) is an index of the airway response to increasing doses of methacholine recorded by whole-body barometric plethysmography.
Keywords: Airway responsivenessMaterials and Reagents
- Sterile saline (0.9% NaCl)
- Methacholine (Sigma-Aldrich, catalog number: A2251 )
- Drierite (Sigma-Aldrich, catalog number: 238988 )
- Nebulization (see Recipes)
Equipment
- Whole body plethysmography system (EMKA Technologies), including standard setup (whole-body plethysmograph, differential pressure transducer, ventilation pump, amplifier, interface box, acquisition card and iox2 software with the respiratory flow analyzer module) and standard nebulisation equipment (LS230, SYSTAM, Villeneuve-sur-Lot)
Procedure
- Turn the plethysmograph system on, and wait for 15 min to let the airflow and signal be stable.
- Control the permeability of each pneumotachograph, and change it for a new pneumotachograph if the permeability is altered.
- Control the dryness of the drierite; dry drierite is blue and turns pink in the presence of humidity. Drierite has to be changed regularly.
- Control functionality of each ventilation pump in the plethysmograph system; each ventilation pump must be regulated to 0.8 L per minute. Check the balls in each flowmeter: All balls have to be perfectly mobile, and show no sign of oxidation (these 3 controls have to be performed before each experiment).
- Turn on the IOX software (delivered with the plethysmograph system).
- Proceed to calibration of each functional plethysmograph chamber.
- Place the animals carefully into each plethysmograph chamber.
- Wait for thirty minutes for the animals to become quiet and calm in the plethysmograph chamber.
- Begin the measurement session.
- Add saline to the nebulizer, start the first nebulisation (30 sec) and record the enhanced pause (PenH) during 20 min.
- Add 0.05 M Methacholine solution, nebulize for 30 sec and record PenH (20 min).
- Add 0.1 M Methacholine solution, nebulize for 30 sec and record PenH (20 min).
- Add 0.2 M Methacholine solution, nebulize for 30 sec and record PenH (20 min).
- Add 0.3 M Methacholine solution, nebulize for 30 sec and record PenH (20 min).
- Save data.
- Turn off the database (IOX software) and the computer.
- Remove each mouse from each chamber.
- Clean all devices.
- Proceed to data analysis. Use the Datanalyst software (delivered with the plethysmograph system) for data collected by the IOX software. The mean PenH of the data measured during 5 min around the peak PenH is used for each dose of methacholine (as shown by the arrows and dotted lines on the Figure 1).
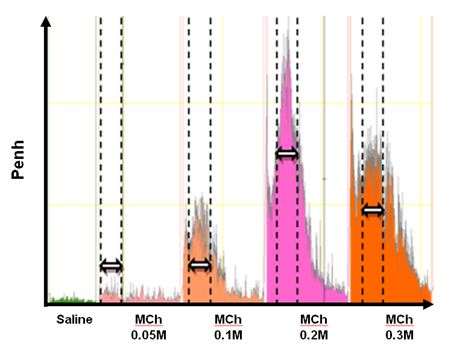
Figure 1.
Recipes
- Nebulization with the standard nebulization system (LS230, SYSTAM) requires 10 ml of each methacholine solution. Prepare a solution of methacholine at 0.3 M, and dilute it to obtain the 0.2 M, 0.1 M and 0.05 M solutions in sterile saline (0.9% NaCl). Methacholine solution has to be prepared on each experimental day from the methacholine powder.
Notes
- If the whole body plethysmograph system is equipped with any other nebulization equipment than LS230 (SYSTAM), we recommend to adapt the methacholine doses.
Acknowledgments
This protocol was adapted from a previously published paper: Reber et al. (2012). FD was supported by the “fond de dotation recherche en santé respiratoire”, call for tenders 2010.
References
- Reber, L. L., Daubeuf, F., Plantinga M., De, Cauwer, L., Gerlo, S., Waelput, W., Van Calenbergh, S., Tavernier, J., Haegeman, G., Lambrecht, B. N., Frossard, N., De Bosscher, K. (2012). A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma. J Immunol 188(7):3478-3487.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Daubeuf, F., Reber, L. and Frossard, N. (2013). Measurement of Airway Responsiveness on Vigil and Unrestrained Mouse. Bio-protocol 3(4): e328. DOI: 10.21769/BioProtoc.328.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









