- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Tumorsphere Formation Assays
Published: Vol 3, Iss 3, Feb 5, 2013 DOI: 10.21769/BioProtoc.325 Views: 56100

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Melanoma Stem Cell Sphere Formation Assay
Alessandra Tuccitto [...] Michela Perego
Apr 20, 2017 15104 Views

Investigating Neural Stem Cell and Glioma Stem Cell Self-renewal Potential Using Extreme Limiting Dilution Analysis (ELDA)
Hong PT Nguyen [...] Theo Mantamadiotis
Sep 5, 2018 11561 Views

3D Co-culture System of Mouse Prostatic Wild-type Fibroblasts with Human Prostate Cancer Epithelial Cells
Veronica R. Placencio-Hickok [...] Neil A. Bhowmick
May 5, 2019 5873 Views
Abstract
A tumorsphere is a solid, spherical formation developed from the proliferation of one cancer stem/progenitor cell. These tumorspheres (Figure 1a) are easily distinguishable from single or aggregated cells (Figure 1b) as the cells appear to become fused together and individual cells cannot be identified. Cells are grown in serum-free, non-adherent conditions in order to enrich the cancer stem/progenitor cell population as only cancer stem/progenitor cells can survive and proliferate in this environment. This assay can be used to estimate the percentage of cancer stem/progenitor cells present in a population of tumor cells. The size, which can vary from less than 50 micrometers to 250 micrometers, and number of tumorspheres formed can be used to characterize the cancer stem/progenitor cell population within a population of in vitro cultured cancer cells and within in vivo tumors (Lo et al., 2012; Liu et al., 2009). While several cell lines can be used for tumorsphere formation assay (e.g. primary mammary tumor cells from Her2/neu-transgenic mice, MCF7, BT474 and HCC1954), some cell lines may not form typical tumorsphere structures and may be difficult to count or classify definitively as tumorspheres.
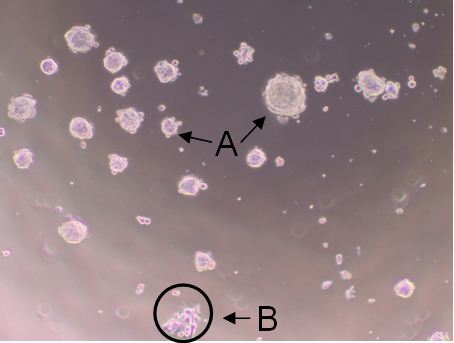
Figure 1. Shows tumorspheres formed byHCC1954 cells after 7 day incubation. The solid, circular formations represent tumorspheres (a). The individual oraggregated cells are not consideredtumorspheres (b).
Note: The tumorspheres should be solid, round structures, but the sizevaries greatly from less than 50 μm to around 250 μm. With aggregated cells, youcan still see individual cells attached to one another. With tumorspheres, thecells appear fused together and it is difficult to distinguish them asindividual cells.
Materials and Reagents
- 50x B27 Supplement (Life Technologies, InvitrogenTM, catalog number: 17504-044 )
- Basic Fibroblast Growth Factor (Sigma-Aldrich, catalog number: F0291 )
- Epidermal Growth Factor (Sigma-Aldrich, catalog number: E5036 )
- Insulin (Life Technologies, InvitrogenTM, catalog number: A11429IJ )
- Bovine Serum Albumin (Sigma-Aldrich, catalog number: A9576 )
- Dulbecco’s Modified Eagle Medium/F12 (Sigma-Aldrich, catalog number: D8437 )
- Sterile 1x Dulbecco’s Phosphate Buffered Saline (Sigma-Aldrich, catalog number: D8537 )
- Trypan Blue (Life Technologies, InvitrogenTM, catalog number: 15250-061 )
- Tumorsphere medium (500 ml) (see Recipes)
Equipment
- Incubator with CO2 input
- Counting Chamber/Hemocytometer (Hausser Scientific, catalog number: 3200 )
- 96-well Ultra-low Attachment Plates (Corning Incorporated, catalog number: 3474 )
Procedure
- Add B27 supplement (50x) to tumorsphere medium for making 1x concentration as needed for experiments. B27 should be freshly added before each experiment.
Note: The addition of B27 has been shown to increase tumorsphere formation and sustain several passages of sphere cultures (Gu et al., 2011). - Harvest cells and suspend the resulting pellet in 5 ml 1x PBS. Keep cells on ice while not in use for the duration of the experiment.
- Gently mix the suspension and pipette 20 μl of the suspension into an eppendorf tube.
- Add 20 μl (1:1 ratio) of Trypan Blue to the cells in the eppendorf tube and mix well.
- Pipette approximately 10 μl of the mixture onto the hemocytometer.
- Count only the bright cells within the four corner quadrants of the counting chamber for viable cell count.
- Take the needed number of cells and add the appropriate volume of tumorsphere medium to make the cell concentration at 1 cell/L. Keep this suspension on ice and mixed well for plating.
- Add 1x PBS to the first and last columns (column 1 and 12) of the 96-well plate to help minimize medium evaporation. This will leave 10 wells available for each row.
- Seed 200 μl of the cells suspended in tumorsphere medium into each well (200 cells per well).
Note: The number of cells used for tumorsphere formation assays may vary with the cell type. - For each cell line or treatment, seed cells into the wells of 2 rows for a total of 20 wells. This will equal a total of 4,000 cells.
- Seal the upper and lower edges of the 96-well plate with laboratory tape to avoid evaporation of medium and place the cells in an incubator set to 37 °C and supply the cells with 5% CO2 for one week.
Note:The medium is not changed or added so as to not disturb the formation of the tumorspheres. - After one-week incubation, tumorsphere numbers are counted under a phase-contrast microscope using the 40x magnification lens (See Figure 1).
- Results can be presented as a percentage of the number of tumorspheres present divided by the initial number of cells seeded (4,000 cells).
Recipes
- Tumorsphere medium (500 ml)
20 ng/ml epidermal growth factor
10 ng/ml basic fibroblast growth factor
5 μg/ml insulin
0.4% Bovine Serum Albumin
500 ml Dulbecco’s Modified Eagle Medium/F12
Acknowledgments
This protocol was first described in Lo et al. (2012). This work was supported by the Elsa U. Pardee Cancer Foundation grant (B94AFFAA), the American Cancer Society Research Award (RSG-10-067-01-TBE) to HC and NIH grant (3P20RR017698-08) to HC and QW.
References
- Gu, Y., Fu, J., Lo, P., Wang, S., Wang, Q., Chen, H. (2011). The effect of B27 supplement on promoting in vitro propagation of Her2/neutransformed mammary tumorspheres. J Biotech Res 3: 7-18.
- Liu, J. C., T. Deng, R. S. Lehal, J. Kim and E. Zacksenhaus (2007). Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res 67(18): 8671-8681.
- Lo, P. K., D. Kanojia, X. Liu, U. P. Singh, F. G. Berger, Q. Wang and H. Chen (2012). CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene 31(21): 2614-2626.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Johnson, S., Chen, H. and Lo, P. (2013). In vitro Tumorsphere Formation Assays. Bio-protocol 3(3): e325. DOI: 10.21769/BioProtoc.325.
Category
Cancer Biology > Cancer stem cell > Cell biology assays > Self-renewal
Cancer Biology > Proliferative signaling > Tumor microenvironment > Proliferation analysis
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









