- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Intracellular Reactive Oxygen Species (CM-H2DCFDA)
Published: Vol 3, Iss 1, Jan 5, 2013 DOI: 10.21769/BioProtoc.313 Views: 90059

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Novel Method for Site-specific Induction of Oxidative DNA Damage to Study Recruitment of Repair Proteins to Heterochromatin and Euchromatin
Leizhen Wei [...] Li Lan
Jun 5, 2014 10324 Views

Measuring Intracellular H2O2 in Intact Human Cells Using the Genetically Encoded Fluorescent Sensor HyPer7
Lianne J. H. C. Jacobs [...] Jan Riemer
Oct 20, 2022 3370 Views

Continuous Measurement of Reactive Oxygen Species Formation in Bacteria-infected Bone Marrow–derived Macrophages Using a Fluorescence Plate Reader
Natascha Brigo [...] Christa Pfeifhofer-Obermair
Feb 5, 2023 2464 Views
Abstract
Reactive oxygen species (ROS) play a critical role in cellular physiopathology. ROS are implicated in cell proliferation, signaling pathways, oxidative defense mechanisms responsible for killing of bacteria, thyroid hormonosynthesis, etc. The cellular Redox homeostasis is balanced by oxidants and antioxidants systems. In several diseases (cancer, neurodegenerative diseases, cardiovascular diseases), the Redox balance is disturbed. In fact, excessive amounts of ROS can damage proteins, lipids and DNA at cellular level.
The choose of a sensitive method for detection of intracellular ROS is very important for detecting the disturbed Redox balance in pathological cells and after exposition of cells to different genotoxic agents (Irradiation, Oxidative stress, etc).
The detection of ROS in biological systems is difficult for several reasons: Method sensibility and probe specificity. The 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) fluorescent probe is commonly employed and may react with several ROS including hydrogen peroxide, hydroxyl radicals and peroxynitrite. The cell-permeant H2DCFDA passively diffuses into cells and is retained in the intracellular level after cleavage by intracellular esterases. Upon oxidation by ROS, the nonfluorescent H2DCFDA is converted to the highly fluorescent 2',7'-dichlorofluorescein (DCF). The chloromethyl derivative of H2DCFDA (CM-H2DCFDA) provides much better retention in live cells than H2DCFDA. Dead or dying cells produces ROS. When we want to detect ROS in living cells, we have to stain cells by propidium iodide (PI) and evaluate ROS production only in living cells which are PI negative. In fact, PI intercalates into double-stranded nucleic acids. It is excluded by viable cells but can penetrate cell membranes of dying or dead cells. PI is excited at 488 nm and emits at a maximum wavelength of 617 nm. Because of these spectral characteristics, PI can be used in combination with other fluorescent probe such as CM-H2DCFDA (excitation/emission: 492–495/517–527 nm).
A probe fluorescence emission can be assessed by Flow cytometry, a standard fluorometer or fluorescence microscopy using appropriate filter.
Flow cytometry is commonly employed to detect intracellular ROS production. Flow cytometry measures fluorescence per cell. The cells is excited by the light source and emitted light from cells are converted to electrical pulses by optical detectors. Emitted Light is send to different detectors by using optical filters: 525 nm Band Pass Filter for FL-1 and 620 nm Band Pass Filter for FL-3. A 525 nm band pass filter (FL-1) placed in the light path prior to the detector will only allow “green” light into the detector. So, FL-1 is used in our protocol to collect green light corresponding to the oxidation of dichlorodihydrofluorescein (DCF) by ROS. FL-1 is the Green (FL-1) channel on flow cytometers. 620 nm Band Pass Filter (FL-3) only allow “red” light into the detector. Red fluorescence emission is measured in the Red (FL-3) channel on most flow cytometers.
Materials and Reagents
- 1x Trypsin-EDTA (0.05% / 0.02% in PBS) (PAA Laboratories GmbH, catalog number: L11-004 )
- DPBS, Calcium, Magnesium (Life Technologies, InvitrogenTM, catalog number: 14040 )
- HBSS Hank’s Balanced Salt Solution (HBSS) with Calcium and Magnesium and without Red Phenol (Life Technologies, InvitrogenTM, catalog number: 14025 )
- CM-H2DCFDA (Life Technologies, InvitrogenTM, catalog number: C6827 )
- Propidium Iodide - 1.0 mg/ml (Life Technologies, InvitrogenTM, catalog number: P3566 )
Equipment
- Cell incubator
- Six-well plates
- Centrifuge
- FACSVantage SE (Becton Dickinson)
- 2 x 75 mm, 5 ml polystyrene round bottom test tube (BD Biosciences, catalog number: 352054 )
Procedure
In the protocol described below, we detect the intracellular ROS after exposure of non cancerous human thyroid epithelial cell line “HTori-3.1” to the oxidative stress. HTori-3.1 cells are grown in complete culture medium (see Recipes).
- HTori-3.1 cells were plated at a density of 2.5 x 105 cells per well into six-well plates in complete culture medium.
- Sixteen hours later, the cells (90% confluence) were washed by warm DPBS, harvested by trypsinization (250 μl of trypsin / Well).
- Add 1 ml of complete culture medium (10% FCS) to stop trypsinization and resuspend cells by gently pipetting up and down.
- Collect cells into 5 ml polystyrene round bottom tube by centrifugation (5 min at 200 x g) at room temperature and aspirate supernatant.
- Wash cell pellet with 4 ml DPBS and centrifuge 5 min at 200 x g at room temperature.
- Resuspend cell pellet in HBSS with 10 μmol/L of CM-H2DCFDA and increased concentration of H2O2 (1 – 5 -10 -15 - 20 - 25 and 50 μmol/L) by gently pipetting up and down (500 μl/tube).
- Incubate cells (resuspended in tubes) in cell incubator [(37 °C), high relative humidity (95%), and controlled CO2 level (5%)] in the dark for 45 min.
- Add Propidium Iodide 0.5 μl/0.5 ml/tube (final concentration = 1 μg/ml) and place the tubes on ice (still in the dark) and proceed immediately to flow cytometry analysis. Note that: a) the propidium iodide staining procedure should be performed immediately before analysis on the flow cytometer, b) when we place tubes on ice we minimize cell activity and we avoid artifactual fluctuations of ROS detection between the first tube analyzed in FACS and following tubes.
- A total of 5,000 events are analyzed in flow cytometry.
- The cellular viability is assessed by propidium iodide staining. Collect PI fluorescence in the FL-3 channel (620 nm) of FACSVantage SE (Becton Dickinson).
- Living cells, which are PI negative, were selected by FACS gating. In these living cells, we record the fluorescence of DCF on FL-1 channel (525 nm) of FACSVantage SE (Becton Dickinson).
Analysis
The effect of H2O2 concentrations from 1 to 50 μmol/L on the intracellular ROS level was determined by flow cytometry analysis. (Figure 1). We can present results as the " all mean fluorescence "or as "M2 percentage" fluorescence variation. M2 indicate the percentage of cells with an increase production of ROS. We measured a concentration-dependent increase of fluorescence, reflecting an increased level of intracellular ROS (Figure 1). 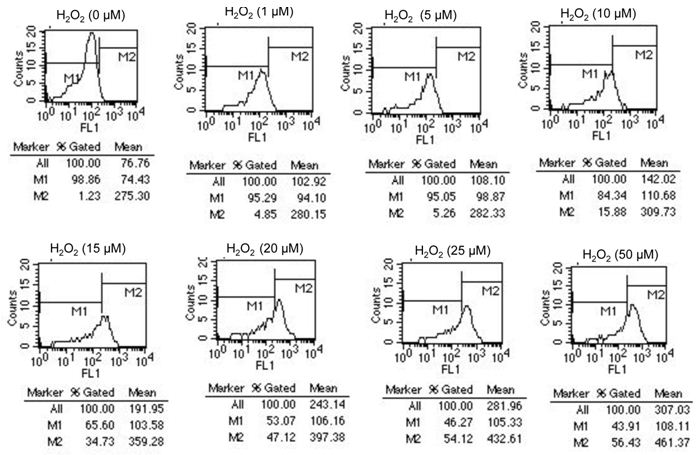
For example, after exposure of cells to 20 μM H2O2, we detect an increase in fluorescence of HTori3.1 cells [Mean fluorescence of all cells is 243.14 in stressed cells and 76.76 in no stressed cells (0 μM H2O2) ]. Our results show that the level of ROS in stressed cells is 3 times more than in no stressed cells.
Also, we can analyze results by "M2 and M1 percentage" fluorescence variation. M1 is placed around the no stressed cells. M2 is placed to the right of M1 to designate positive events. We look at the %Gated for M2: 1.23% (no stressed cells) and 47.12% (cells stressed with 20 μM H2O2). Our results indicate that 47,12% of cells show an increase in ROS level after exposure to 20 μM H2O2.
Recipes
Complete culture medium: phenol red–free RPMI 1640 (Life Technologies, InvitrogenTM), supplemented with 1% (v/v) antibiotics/antimycotics (Life Technologies, InvitrogenTM), 2 mmol/L of L-glutamine (Life Technologies, InvitrogenTM), and 10% (v/v) FCS (PAA Laboratories).
Acknowledgments
This protocol is adapted from Ameziane-El-Hassani et al. (2010).
References
- Ameziane-El-Hassani, R., Boufraqech, M., Lagente-Chevallier, O., Weyemi, U., Talbot, M., Metivier, D., Courtin, F., Bidart, J. M., El Mzibri, M., Schlumberger, M. and Dupuy, C. (2010). Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res 70(10): 4123-4132.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ameziane-El-Hassani, R. and Dupuy, C. (2013). Detection of Intracellular Reactive Oxygen Species (CM-H2DCFDA). Bio-protocol 3(1): e313. DOI: 10.21769/BioProtoc.313.
Category
Cell Biology > Cell staining > Reactive oxygen species
Biochemistry > Other compound > Reactive oxygen species
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









