- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sphere Formation (Osteosphere/Sarcopshere) Assay
Published: Vol 2, Iss 24, Dec 20, 2012 DOI: 10.21769/BioProtoc.307 Views: 21940

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Melanoma Stem Cell Sphere Formation Assay
Alessandra Tuccitto [...] Michela Perego
Apr 20, 2017 15104 Views

Investigating Neural Stem Cell and Glioma Stem Cell Self-renewal Potential Using Extreme Limiting Dilution Analysis (ELDA)
Hong PT Nguyen [...] Theo Mantamadiotis
Sep 5, 2018 11564 Views
Abstract
Self-renewing cells from adult tissue (such as bone) that represent a progenitor population can be grown in suspension cultures in the presence of defined serum-free medium. Progenitor cells can be identified by this property of anchorage-independent growth in suspension cultures. These spherical clusters of progenitor bone cells growing under non-adherent conditions are called osteospheres. Such progenitor populations often possess characteristics of multipotency and can differentiate into multiple mesenchymal lineages. Cancer cells capable of growing in suspension have also been reported in osteosarcomas, tumors of the bone tissue. These spherical colonies formed from single cells (clonal) in non-adherent conditions are generally considered to represent self-renewing, stem-like cells and can be employed for other assays such as multipotency and limiting dilution analysis (LDA).
Materials and Reagents
- Osteosarcoma cells (Basu-Roy et al., 2012)
- Trypsin
- Trypan blue from Invitrogen (Life Technologies, Invitrogen™, catalog number: 15250-061 )
- N2B27-defined serum free medium
- DMEM/F-12 1:1 from Invitrogen (Life Technologies, Invitrogen™, catalog number: 11330-057 )
- Neurobasal medium from Invitrogen (Life Technologies, Invitrogen™, catalog number: 21103-049 )
- Glutamax – from Invitrogen (Life Technologies, Invitrogen™, catalog number: 35050-061 )
- 55 mM Beta-mercaptoethanol – from Invitrogen (Life Technologies, Invitrogen™, catalog number: 21985-023 )
- B27 serum-free supplement – from Invitrogen (Life Technologies, Invitrogen™, catalog number: 17504-044 )
- Ndiff Neuro-2-medium supplement from Millipore (EMD Millipore, catalog number: SCM012 )
- DMEM/F-12 1:1 from Invitrogen (Life Technologies, Invitrogen™, catalog number: 11330-057 )
- Complete N2B27-medium (see Recipes)
Equipment
- Standard tissue culture equipment
- Ultra-low attachment tissue culture plastic ware from Corning (Corning Incorporated, catalog number: 3473 for 24 well plate; catalog number: 3474 for 96 well plate)
- 40 μM cell trainer from BD Falcon (BD Biosciences, Falcon®, catalog number: 352340 )
- Dissecting microscope
- Accumax (Innovative Cell Technologies, model: AM 105 )
- Hemocytometer
- 10-cm plate (Corning Incorporated, catalog number: 3262 )
Procedure
- Plating cells for sphere formation assay
- Trypsinize log-phase (actively growing) osteosarcoma cells (or cell of interest) growing under adherent conditions, and neutralize trypsin with serum-containing medium and count live cells after Trypan Blue staining, using a hemocytometer.
- Take required amount of cell suspension, spin down and resuspend cells at 1,000 live cells ml in N2B27-defined medium. Plate 1 ml cell suspension (1,000 cells) per well in triplicate in 24-well Corning ultra-low attachment plates for 7-10 days in N2B27 defined serum free medium.
Note: Cell density is critical since too many cells will lead to clumping (and false positive results) and too few cells will not lead to sphere formation. We recommend seeding multiple densities as a pilot experiment to empirically determine best plating cell density before proceeding with the actual experiment. Cell numbers presented here have worked well in our osteosarcoma system. Also make sure that cells are single-cell suspension before plating by checking under microscope. - Feed cells every 3 days by adding 50-100 μl medium per well. Do not aspirate medium since medium change is usually not required. Medium lost through evaporation is replenished during feeding. Also, be careful while feeding spheres since vigorous pipetting tends to disassociate the spheres.
- Check for sphere formation and count spheres in each well after 7-10 days.
Note: Some cells especially non-transformed cells may require longer incubation of 2-3 weeks to form countable spheres. Spheres can be distinguished from clumps since spheres are uniformly circular whereas clumps look like aggregates that are not uniform in appearance.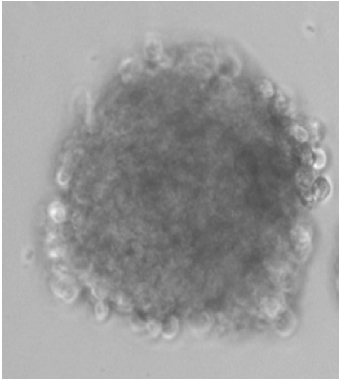
Figure 1. A typical sphere generated from osteosarcoma cells - Count spheres under a dissecting microscope. These spheres are called primary spheres.
Note: Typically, we see 0.1-0.5% of primary cells plated form spheres and 1-5% of transformed cells plated form spheres. Sphere forming frequency is determined by dividing the total number of spheres by the number of cells plated. - After counting spheres under dissecting microscope, collect primary spheres by passing through a 40 μm cell strainer. Wash the plate once with PBS and pass the wash through the strainer to collect residual spheres. Do not forget to mention that you have collected spheres whose size is > 40 μm in your methods section.
Note: Alternatively, you can plate more cells (10,000 cells/ml) - 10 ml cell suspension/10-cm plate or use the same cell density but plate higher volumes, and collect the spheres if you need more spheres. It should be stated here that you might need to increase the number of cells/ml if you plate higher volumes since autocrine and paracrine factors that are secreted by cells get diluted in higher volumes and hence, higher cell numbers might need to be used to compensate for this dilution. - After collection of spheres, they can be used for various downstream applications such as protein extraction, RNA isolation, colony assay and re-plating for secondary sphere formation.
- For re-plating for colony or secondary sphere formation assay, primary spheres can be dissociated into single cells using Accumax according to the manufacturer's protocol. Before re-plating for colony or secondary sphere formation assay, check viability of cells by doing Trypan blue dye exclusion and counting cells using a hemocytometer.
- Trypsinize log-phase (actively growing) osteosarcoma cells (or cell of interest) growing under adherent conditions, and neutralize trypsin with serum-containing medium and count live cells after Trypan Blue staining, using a hemocytometer.
Recipes
- Complete N2B27-medium
To make complete N2B27-medium, mix
200 ml DMEM/F-12
200 ml Neurobasal medium
1 ml Ndiff Neuro-2-medium supplement
2 ml Glutamax
4 ml B27 supplement
4 ml Penicillin/Streptomycin
728 μl of Beta-mercaptoethanol
Acknowledgments
This investigation was supported by PHS Grants AR051358 from the NIAMS and DE013745 from the NIDCR, and by an NCI UO1 award (to SHO). UBR is a recipient of a fellowship from The Children's Cancer Research Fund in memory of Dr A Rausen. AM is a recipient of a research grant from St Baldrick's Foundation. JAP is a postdoctoral fellow of the American Cancer Society. SHO is an Investigator of the Howard Hughes Medical Institute. This protocol is associated with the manuscript Basu-Roy et al. (2012).
References
- Basu-Roy, U., Ambrosetti, D., Favaro, R., Nicolis, S. K., Mansukhani, A. and Basilico, C. (2010). The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ 17(8): 1345-1353.
- Basu-Roy, U., Seo, E., Ramanathapuram, L., Rapp, T. B., Perry, J. A., Orkin, S. H., Mansukhani, A. and Basilico, C. (2012). Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 31(18): 2270-2282.
Article Information
Copyright
© 2012 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Basu-Roy, U., Basilico, C. and Mansukhani, A. (2012). Sphere Formation (Osteosphere/Sarcopshere) Assay. Bio-protocol 2(24): e307. DOI: 10.21769/BioProtoc.307.
Category
Cancer Biology > Cancer stem cell > Cell biology assays > Self-renewal
Cancer Biology > General technique > Cell biology assays > Cell viability
Stem Cell > Adult stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










