- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishing Primary Malignant Pleural Mesothelioma (MPM) Cell Cultures
Published: Vol 2, Iss 21, Nov 5, 2012 DOI: 10.21769/BioProtoc.285 Views: 15415

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Murine Alveolar Type II Epithelial Cells
Fan Sun [...] Zhaoxia Qu
May 20, 2017 13115 Views

Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis
Feng Du [...] Daiming Fan
Jun 20, 2017 30589 Views

A Fast and Reliable Method to Generate Pure, Single Cell-derived Clones of Mammalian Cells
Zhe Han [...] Varun Kumar
Aug 20, 2022 4105 Views
Abstract
This is a general protocol for the isolation and maintenance of primary MPM cultures as a tool for the identification of Tumor Initiating Cells and early progenitor-targeting drugs (Cioce et al., 2010). Primary cultures can be propagated efficiently for 8-12 weeks and xenotransplanted in NOD/SCID mice while retaining the histofeatures of the originating tumor (Canino et al., 2012). The protocol is suitable for both MPM solid specimens and pleural effusion. For increased clarity, initially two separate sections addressing the isolation of MPM cells from solid tumors and pleural effusions are here provided.
Materials and Reagents
- Surgical specimen or pleural effusion
- Phosphate buffered saline (PBS)
- Collagenase type XI (300 U/ml) (Sigma-Aldrich, catalog number: C9407 )
- Hyaluronidase (100 U/ml) (Sigma-Aldrich, catalog number: H4272 )
- DMEM/F12 (1:1)+ GLUTAMAX (Life Technologies, Invitrogen™, catalog number: 10565-018 )
- FBS-non heat inactivated (Life Technologies, catalog number: 10437-028 )
- Human recombinant insulin (Sigma-Aldrich, catalog number: I-5500 )
- Bovine Serum Albumin-Fatty Acid Free (BSA-FAF) (Sigma-Aldrich, catalog number: A7030 )
- Non-heat inactivated FBS (Life Technologies, Gibco®, catalog number: 16000-044 )
- Ciprofloxacin (Sigma-Aldrich, catalog number: 17850 )
- Red blood lysis buffer (0.8% ammonium chloride) (Life Technologies, catalog number: A10492-01 )
- ACCUTASE (STEMCELL Technologies, catalog number: AT-104 )
- 0.4% trypan blu (Life Technologies, Gibco®, catalog number: 15250-061 )
- Digestion medium
Equipment
- Cell culture set up
- Scalpels (Becton, Dickinson and Company, catalog number: 371621 )
- Microdissecting forceps
- 5 ml Pasteur pipette (BD Biosciences, Falcon®)
- 15 ml centrifuge tubes
- 50 ml centrifuge tubes
- Centrifuge capable of running at ≥ 300 x g
- 70 μm nylon mesh (BD Biosciences, Falcon®, catalog number: 352350 )
- Ultralow attachment dishes (Corning Incorporated, catalog number: 3261 for 100 mm) (or alternatively, sterile Petri dishes non treated for cell culture)
Procedure
- Protocol 1A. Procedure for isolating MPM cells from surgical specimens
Critical step: The time interval from tumor resection and digestion must be kept to a minimum, ideally ≤ 1 h.
Critical step: To prevent undesired contamination, work under sterile condition at all steps and whenever possible.- Wash the tumor specimen twice with PBS 1x supplemented with Ciprofloxacin (4 μg/ml) (2 x 40 ml in a 50 ml tube). To disaggregate the solid tumor follow three sequential steps (in a tissue-culture sterile hood):
- Manually cut the solid tumor into ≤ 1mm pieces with scalpels in a sterile Petri dish with 1 ml 1x PBS.
- Enzymatic disaggregation: Re-suspend tumor pieces in a 100 mm cell culture dish with 5 ml of digestion medium (DMEM-F12 + GLUTAMAX supplemented with 1% BSA-FAF and 5 μg/ml human insulin). After resuspension, add collagenase (final concentration 30 U/ml) and hyaluronidase (final concentration 10 U/ml) to the tumor suspension and leave cells in the incubator for 2 h at 37 °C, 5% CO2. Every 15 min re-suspend the semi-digested tumor with a 5 ml sterile Pasteur pipette by gently pipetting up and down to disperse tumor pieces.
- Manually cut the solid tumor into ≤ 1mm pieces with scalpels in a sterile Petri dish with 1 ml 1x PBS.
- Filter the digested material through a 70 μm nylon mesh in a 50 ml tube.
- Transfer the filtered material to a 15 ml centrifuge tube.
- Spin at 300 x g for 10 min at room temperature (RT).
- Re-suspend the pellet in fresh medium DMEM F12 (1:1) + GLUTAMAX supplemented with 5% non-heat inactivated FBS, insulin (10 μg/ml) and ciprofloxacin (4 μg/ml). Count total live cell number with Trypan-Blue exclusion method.
- Seed cells in low-adhesion cell culture dishes at a cell density ≥ 1-1.5 x 106 cells/ml). Growth cells for 3 weeks and add 25% fresh medium once a week. After 3 weeks, a quite homogeneous population of mesothelioma cells can be observed in culture. These can be propagated (to a limited extent-see below) or harvested for further processing.
- Wash the tumor specimen twice with PBS 1x supplemented with Ciprofloxacin (4 μg/ml) (2 x 40 ml in a 50 ml tube). To disaggregate the solid tumor follow three sequential steps (in a tissue-culture sterile hood):
- Protocol 1B. Procedure for isolating MPM cells from pleural effusions
- Collect the pleural effusion in 15 ml FALCON tubes diluted 1:1 with 1x PBS supplemented with Ciprofloxacin (4 μg/ml).
- Harvest cells by centrifugation at 300 x g for 10 min at room temperature (RT). Keep the cell-free medium (supernatant - pleural effusion).
- Resuspend cells in Red Blood Lysis buffer (10 bed pellet volumes). Incubate for 5 min at room temperature (RT).
- Centrifuge (300 x g, 10 min) and discard supernatant.
- Re-suspend the pellet in fresh medium DMEM F12 (1:1) + GLUTAMAX supplemented with 5% non-heat inactivated FBS, insulin (10 μg/ml) and ciprofloxacin (4 μg/ml) + 30% cell free medium (as from step b). Count total live cell number with Trypan-Blue exclusion method.
- Seed cells in low-adhesion cell culture dishes at a cell density ≥ 1-1.5 x 106 live cells/ml. Size of the dish must be chosen according to the available number of cells in order to achieve the desired concentration.
- Grow cells for 3 weeks and add 25% fresh medium once a week. After an average of 3 weeks, a heterogeneous population of mesothelioma cells can be observed in culture. This is mainly composed of adherent and loosely attached cells (Figure 1), which can be propagated (to a limited extent-see below) or harvested for further processing.
- Collect the pleural effusion in 15 ml FALCON tubes diluted 1:1 with 1x PBS supplemented with Ciprofloxacin (4 μg/ml).
- Protocol 2. Propagation of MPM cell cultures
For the propagation of the primary cell cultures, always collect both floating (or loosely adherent) and adherent cell subpopulations.
It is very important to keep conditioned medium during harvesting of the cells and add it back to cell culture during the establishment of the culture (Protocol 1, b) or during propagation of the cells (Protocol 2, a).
All the volumes listed below refer to 100 mm dishes. Please vary volumes of solutions according the size of the cell culture dishes used.- Collect cell culture medium in a centrifuge tube. Wash the adherent cells with 3-5 ml of 1x PBS and add it to the collected cell culture medium. This contains loosely adherent or floating cells (1x PBS should be ≤ 30% final volume in the collection tube).
- Wash cells again with 1x PBS, discard 1x PBS and add accutase (1.5 ml/dish).
- Incubate cells in the incubator for 5 min at 37 °C, 5% CO2.
- Harvest Accutase-treated (detached) cells with the collected cell culture medium/PBS 1x washing from step a. Count total live cell number with Trypan-Blue exclusion method.
- Seed cells in low-adhesion cell culture dishes at a density ≤ 0.5 x 106 live cells/ml). To achieve the required cell concentrations dilute the harvested cells with DMEM F12 (1:1) + GLUTAMAX supplemented with 5% non-heat inactivated FBS, insulin (20 μg/ml) and ciprofloxacin (4 μg/ml). The dilution medium must represent ≥ of the 50% of the final cell culture volume in the dish.
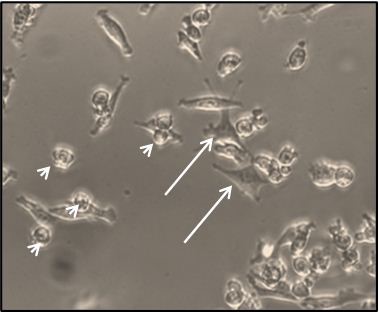
Figure 1. Representative micrograph of a MPM cell culture (from a malignant pleural effusion) at 2 weeks after seeding. Arrows: adherent, fibroblast-like cells. Arrowheads: loosely adherent, rounded cells.
- Collect cell culture medium in a centrifuge tube. Wash the adherent cells with 3-5 ml of 1x PBS and add it to the collected cell culture medium. This contains loosely adherent or floating cells (1x PBS should be ≤ 30% final volume in the collection tube).
Notes
- Ciprofloxacin prevents contamination of the material from non-sterile handling of the tumor specimens during the harvesting of the sample.
- The obtained tumor digests consist initially of a heterogeneous population, comprising but not limited to Mesothelioma cells, macrophages, Immune infíltrate, stromal cells, adipocytes and remnant red blood cells. However, within 72-96 h from seeding most of the cells in culture consist of mesothelioma cells, since the mentioned accompanying cell subpopulations will not propagate in the experimental conditions used here, as revealed by morphological observations and clonogenic assays (Canino et al., 2012) The obtained populations have been shown to origínate MPM-like tumors when injected into NOD/SCID mice with very high resemblance to the originating tumor (Canino et al., 2012).
- A typical yield of 1 x 106 cells can be obtained from a 100 mg solid specimen. A typical yield of 10 x 106 cells can be obtained from 30 to 5 ml freshly collected pleural effusion (after red blood lysis removal).
Acknowledgments
This protocol was adapted from Canino et al. (2012). We acknowledge funding from INAIL (Italian Workers Compensation Authority) and AIRC-ROC and to GB.
References
- Canino, C., Mori, F., Cambria, A., Diamantini, A., Germoni, S., Alessandrini, G., Borsellino, G., Galati, R., Battistini, L., Blandino, R., Facciolo, F., Citro, G., Strano, S., Muti, P., Blandino, G. and Cioce, M. (2012). SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene 31(26): 3148-3163.
- Cioce, M., Gherardi, S., Viglietto, G., Strano, S., Blandino, G., Muti, P. and Ciliberto, G. (2010). Mammosphere-forming cells from breast cancer cell lines as a tool for the identification of CSC-like- and early progenitor-targeting drugs. Cell Cycle 9(14): 2878-2887.
Article Information
Copyright
© 2012 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cioce, M., Canino, C., Strano, S. and Blandino, G. (2012). Establishing Primary Malignant Pleural Mesothelioma (MPM) Cell Cultures. Bio-protocol 2(21): e285. DOI: 10.21769/BioProtoc.285.
Category
Cancer Biology > General technique > Cell biology assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









