- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mandibular Explant Assay for Investigating Extrinsic Stimuli on Bone and Cartilage Development
Published: Vol 7, Iss 23, Dec 5, 2017 DOI: 10.21769/BioProtoc.2641 Views: 7925
Reviewed by: Alessandro DidonnaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1833 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3776 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2638 Views
Abstract
A major issue in developmental biology is to determine how embryonic tissues respond to molecular signals in a timely manner and given the position-restricted instructions during morphogenesis, of which Meckel’s cartilage is a classical example. The ex-vivo explant model is a practical and convenient system that allows investigation of bone and cartilage responses to specific stimuli under a controlled manner that closely mimics the in vivo conditions. In this protocol, the explant model was applied to test whether Meckel’s cartilage and surrounding tissues are responsive to the Endothelin1 (Edn1) signaling molecule and whether it would rescue the phenotype of genetic mutations. The system allows a high degree of manipulation in terms of the concentrations of exogenous compounds added to the explant, time points with regards to measuring mandibular development, and the method of application of exogenous molecules and teratogens.
Keywords: Ex-vivo mandibular explantBackground
Craniofacial malformations are among the most frequent congenital birth defects in humans (Miettinen et al., 1999). Many of these malformations occur during facial morphogenesis, a complex multi-step process in which cranial neural crest cells migrate to pharyngeal arches to give rise to many facial structures (Jin et al., 2011).
Both variations within specific genes as well as gene-gene interactions can lead to craniofacial deformations. Mutations in IRF6, a gene that contributes to the formation of ectoderm and epithelium in the head and face, can result in cleft lip, cleft palate, and mandibular abnormalities. In comparison to IRF6, the TWIST1 gene regulates neural tube closure during embryonic development and cranial suture fusion during skull development. Mutations in TWIST1 can cause craniosynostosis, mandibular hypoplasia, and cleft palate (Fakhouri et al., 2017). Inhibition or alteration of IRF6 and TWIST1 expression can be done similar to the methods performed by Miettinen et al. (1999) with EGF receptors in order to examine their roles in craniofacial development. However, difficulties arise in in vivo experiments when the study begins to incorporate genetic interactions and rescue experiments of two or more allelic mutations. In our recent study, the genetic interaction between Irf6 and Twist1 causes severe mandible abnormality and cleft of the secondary palate in the mouse model (Fakhouri et al., 2017).
Various studies have used in vivo experiments to test for the expression of various genes involved in craniofacial development. The presence of TGF-β subtypes was studied by using an ex vivo culture model in a serumless, chemically defined medium during mandibular morphogenesis (Chai et al., 1994). Similarly, a study used Alcian blue staining of cultured mandible explants to examine Meckel’s cartilage during morphogenesis in Egfr-/- embryos (Miettinen et al., 1999). A combination of the methodologies from these studies, including an ex vivo mandibular explant described in this report, is useful to characterize the phenotype and signaling pathway in mammalian systems.
Materials and Reagents
- 60 mm TC-Treated center-well organ culture dish (Corning, Falcon®, catalog number: 353037 )
- Petri dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 130182 )
- Test tubes (15 and 50 ml) (Denville Scientific, catalog numbers: C1012 and C1062-P , respectively)
- Stainless steel grid (0.5 mm hole size) (Home Depot International, model: 3004107 , catalog number: 1001034564)
- Millipore type filters (0.8 µm pore size, 47 mm diameter) (Merck, catalog number: AAWP04700 )
- Sterile individually packaged pipettes (2, 5, and 10 ml) (Genesee Scientific, catalog numbers: 12-101 , 12-102 , 12-104C )
- E10.5-E11.5 murine embryos
- 70% ethanol
- 95% ethanol
- 6-Aminonicotinamide (Alfa Aesar, catalog number: L06692 )
- 2 mm-diameter agarose beads or Affi-Gel blue gel (Bio-Rad Laboratories, catalog number: 732-6712 )
- Endothelin1 peptide (Enzo Life Sciences, catalog number: ALX-155-001-PC01 )
- Thymol (Sigma-Aldrich, catalog number: T0501 )
- Sodium phosphate dibasic heptahydrate (Na2HPO4·7H2O) (Sigma-Aldrich, catalog number: S9390 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Potassium chloride (KCl) (Fisher Scientific, catalog number: BP366 )
- Potassium phosphate monobasic (KH2PO4) (Fisher Scientific, catalog number: P286-1 )
- BGJb medium with L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 12591038 )
- Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F0926 )
- Ascorbic acid (Fisher Scientific, catalog number: S25184 )
- Penicillin-streptomycin 100x (Caisson Laboratories, catalog number: PSL01-100ML )
- MEM non-essential amino acid solution 100x (Sigma-Aldrich, catalog number: M7145 )
- Glacial acetic acid (Fisher Scientific, catalog number: S25118A )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P1767 )
- Alcian Blue 8GX (National Diagnostics, catalog number: HS-504 )
- Alizarin Red S (Acros Organics, catalog number: 400480250 )
- Glycerol (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 17904 )
- Nuclear fast red (Acros Organics, catalog number: 211980050 )
- 10x phosphate-buffered saline (PBS) (see Recipes)
- Mandibular explant media (see Recipes)
- 3% acetic acid solution (see Recipes)
- 2% KOH solution (see Recipes)
- Alcian blue solution pH 2.5 (see Recipes)
- Alizarin red solution (see Recipes)
- 1% KOH / 20% glycerol solution (see Recipes)
Equipment
- Surgical scissors (World Precision Instruments, catalog number: 501225 )
- Sterile cell culture hood (NuAire, model: NU-545 )
- Stereomicroscope (Nikon Instruments, model: SMZ800N )
- Forceps (World Precision Instruments, catalog number: 15915 )
- Autoflow IR Direct Heat CO2 incubator (NuAire, model: NU-5510 )
- Ceramic hot plate (VWR, catalog number: 97042-602 )
- Pipette pump (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 9511 )
- Autoclave (Future Health Concepts, Primus, catalog number: PRPSS8-A )
Software
- NIS Elements AR software (Nikon Instruments) used for the stereomicroscope
Procedure
- Embryos (E10.5-E11.5) extraction
- Perform euthanization via CO2 followed by cervical dislocation on a pregnant female mouse (see Note 1). All animal procedures were approved by the Animal Welfare Committee (AWC-16-0068) at the University of Texas Health Science Center at Houston and followed the National Institute of Health guidelines as described by Metwalli et al., 2017.
- Transfer the animal to a clean procedural room.
- Spray ethanol (70%) on the abdominal area of the female.
- Use scissors to cut the abdominal skin from the lower abdomen to the epigastric region. Perform the cut at the midline of the abdomen.
- Pull the uterus out of the body and cut the connective tissue. This will separate the uterus from the fallopian tubes and ovaries.
- Keep all embryos together in the uterus sack.
- Place the sack in a Petri dish with cold 1x PBS (15-20 ml, see Recipe 1).
- In a sterile hood, separate the embryos by cutting the uterus between the embryos.
- Transfer all embryos to a new Petri dish with 1x PBS (15-20 ml) at RT to wash and remove excess blood.
- Open the amniotic sac and separate the placenta from the embryos, severing the umbilical cord near the embryo.
- Place the embryo in a 15 ml test tube.
- Wash each embryo twice with 5 ml of 1x PBS (see Note 2).
- Perform euthanization via CO2 followed by cervical dislocation on a pregnant female mouse (see Note 1). All animal procedures were approved by the Animal Welfare Committee (AWC-16-0068) at the University of Texas Health Science Center at Houston and followed the National Institute of Health guidelines as described by Metwalli et al., 2017.
- Mandibular extraction
- In a sterile cell culture hood, dissect the mandibular processes of the first pharyngeal arch under a stereomicroscope while in BGJb culture medium at RT.
- Pre-warm BGJb culture medium and mandibular explant media (see Recipe 2) at 37 °C. Add 1-2 ml of PBS at RT to the exterior portion of the center-well organ dish and 0.5 ml of the mandibular explant media to the center-well of the organ dish.
- Orient the embryos in a supine position. Using a scalpel, cut through the labial commissure of the mouth at each corner of the mouth in order to separate the mandibular process from the head of the embryo (see Figure 1).
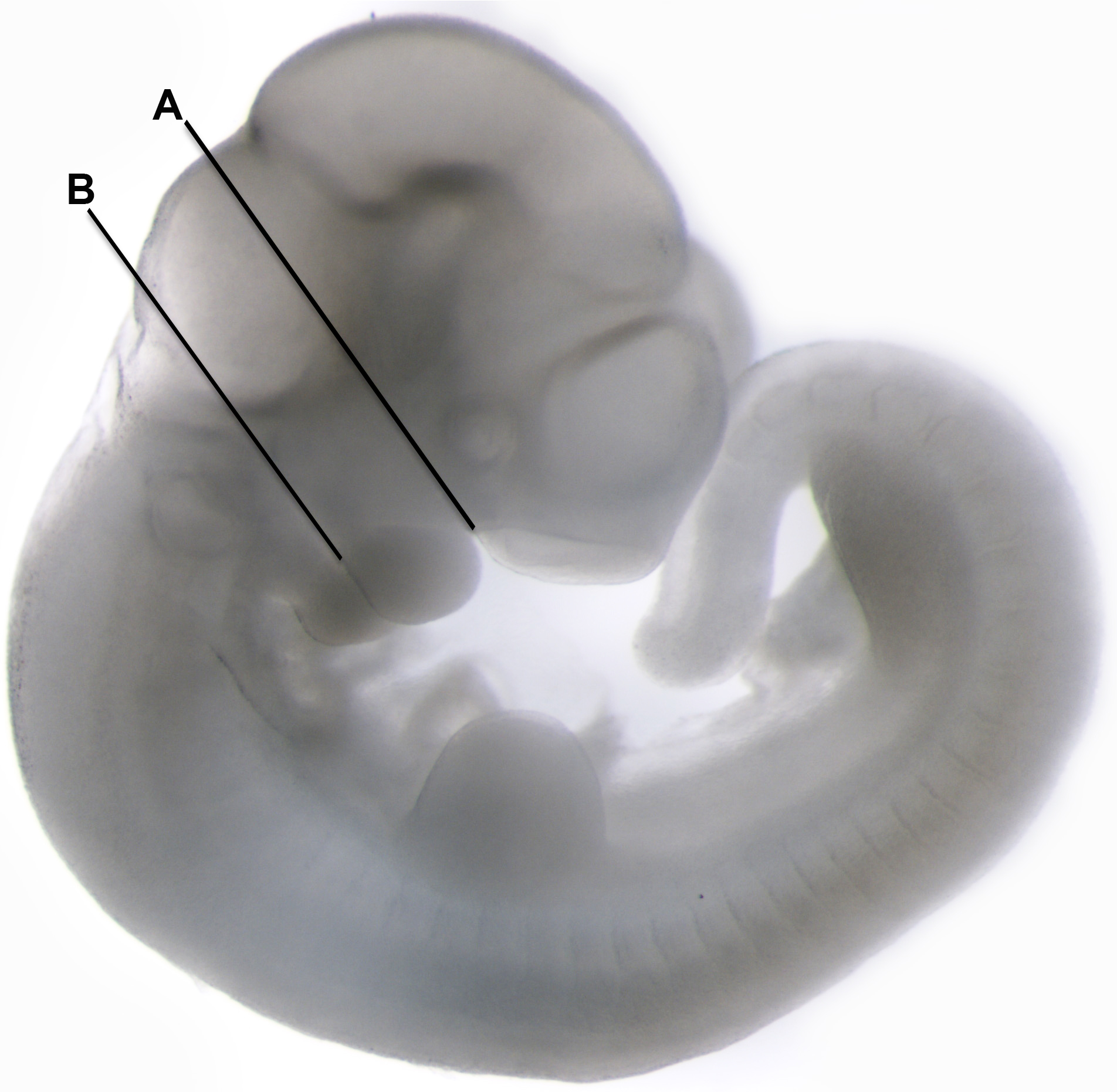
Figure 1. A murine embryo at E11.5 used for dissecting the mandibular process for ex-vivo assay. The first cut was done at the commissure of the mouth all the way to the hind-brain (A). The second cut was performed at the top of the neck to separate the mandibular process from the rest of the body (B).
- Make another cut through each embryo’s neck using a scalpel to completely separate the mandibular process from the rest of the embryo.
- Place a single stainless steel mesh across the center-well organ dish, so it is submerged in the mandibular explant media (see Figure 2 and see Note 3).
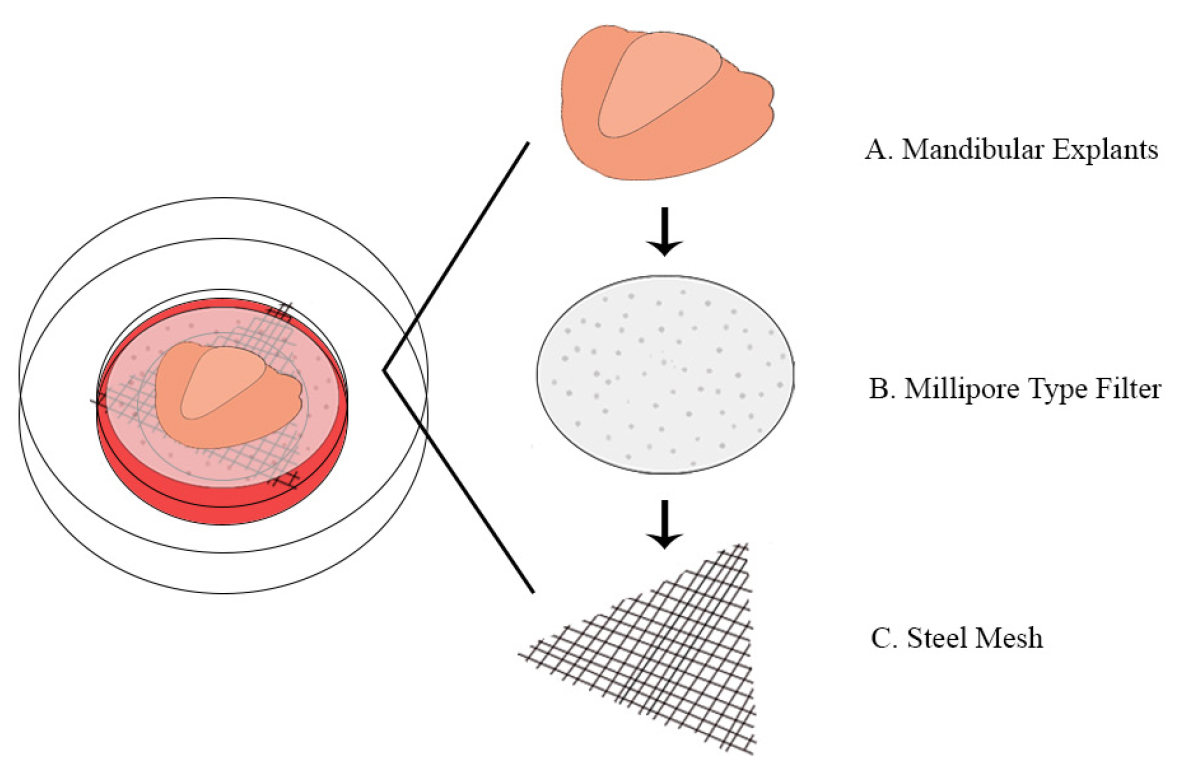
Figure 2. Design of mandibular explant on organ-well culture plate
- Lay filter paper on top of the metal mesh sheet and allow the filter paper to be submerged in the mandibular explant media (see Note 4).
- Place explants in the center of the filter paper.
- Place the Petri dish in a CO2 incubator at 37 °C.
- Replace the media (0.5 ml) in the center-well with pre-warmed (37 °C) fresh mandibular explant media every 12 h for the first 24 h and then once a day after the first 24 h.
- After 12 h of incubation, treat the mandibular sections with 100 mg/ml of 6-aminonicotinamide in mandibular explant media for 3 h. In our study, the 6-aminonicotinamide treatment was used in the experimental samples as a teratogen to the explant (see Note 5) in order to disturb proper tissue development.
- After 3 h treatment, replace the media containing the teratogen with fresh pre-warmed (37 °C) mandibular explant media.
- In our case, after the teratogen treatment, the experimental mandible samples were treated with 2 mm-diameter agarose beads containing 0.1 μg/ml of endothelin-1 (Edn1) peptide for 8 h in the CO2 incubator at 37 °C (see Note 6). The Edn1 was applied to test whether Meckel’s cartilage and the surrounding tissues are responsive to this signaling molecule and if it would rescue the phenotype of genetic mutations.
- After the 8 h treatment, remove the media and the agarose beads that contained Edn1 peptide.
- Replace the media (0.5 ml) in the center-well with pre-warmed (37 °C) fresh mandibular explant media. After the first 24 h, replace the media once a day (see Note 7).
- After 24-120 h, fix explants with 95% ethanol for 1-2 days at RT.
- Replace the 95% ethanol with 2% KOH (see Recipe 4) for 2-4 h until the explant tissue becomes transparent.
- Replace 2% KOH with Alcian Blue staining overnight at RT (see Recipe 5). Alcian Blue stains for acidic glycosaminoglycans found in cartilage.
- Replace staining with 95% ethanol for 12-24 h at RT.
- Replace the 95% ethanol with Alizarin Red staining (see Recipe 6) for 6 h (Figure 3) at RT. Alizarin Red will stain for calcium containing osteocytes.
- Submerge explants in 20% glycerol and 1% KOH (see Recipe 7) to remove excess staining.
- Replace solution with 100% glycerol to store at 4 °C. An antifungal such as Thymol may be added to the storing solution (optional).

Figure 3. Ex-vivo mandibular explant models at E11.5. A. Embryonic mandibular explants extracted at E11.5 and cultured for two days. The image was taken from a side view. B. Alcian Blue stained mandibular explants for Meckel’s cartilage two days post-incubation. The image was taken from the top view of the mandible. C. Alcian blue and Alizarin red staining of mandibular explants after 4 days of incubation.
- In a sterile cell culture hood, dissect the mandibular processes of the first pharyngeal arch under a stereomicroscope while in BGJb culture medium at RT.
Data analysis
Skeletal staining was performed on the explants and observed through the stereomicroscope using the software NIS Elements AR. For our study, we examined changes in cartilage and bone formation. In this case, we observed variations including a lack of symmetry, arrested growth of the cartilage, and the shape and thickness of the Meckel’s cartilage. It is recommended to use at least five technical replicates, meaning five embryos from the same litter, for each experimental treatment to account for any intrinsic variation.
Notes
- The age of embryos desired to be extracted can be determined by the date of breeding with male and the presence of a copulation plug in the pregnant female.
- Each embryo should be washed at least twice. If blood remains, the embryos should be washed until all the blood is removed. The embryos must remain wet during all manipulation steps.
- The steel mesh was cut into 1 cm by 1 cm triangles by a guillotine in order to be placed on top of the media in the inner well of the organ culture dish. In our case, the steel mesh was sterilized by dousing it in ethanol and placing it on a fire.
- The Millipore filter paper was cut into 1 cm squares, in order to fit within the inner well of the organ culture dish.
- 6-Aminonicotinamide was directly added to the medium prior to treatment with Edn1 ligand. Explants without pre-treatment of 6-aminonicotinamide and only treated with Edn1 were included as controls.
- Edn1 was delivered by direct pipetting on 2-mm agarose beads and incubated for 5 min. The beads containing Edn1 were placed on either side of the mandibular explants by forceps, and left for 8 h in order for the molecules to diffuse out.
- The mandibular explants may be kept in culture for up to one week without any noticeable necrosis or deterioration.
- All the tools and solutions used in this protocol should be sterile.
Recipes
- 10x phosphate-buffered saline (PBS)
25.6 g Na2HPO4·7H2O
80 g NaCl
2 g KCl
2 g KH2PO4
Bring to 1 L with distilled H2O and dissolve the salts by using the ceramic plate and magnetic stir bar
For 1x PBS, dilute 100 ml of 10x PBS in 900 ml of distilled H2O. Sterilize by autoclaving
- Mandibular explant media
47.5 ml of BGJb medium supplemented with L-glutamine
3% FBS (1.5 ml/50 ml medium)
7 mg of ascorbic acid
0.5 ml of streptomycin-penicillin solution (100x)
0.5 ml of MEM non-essential amino acid solution (100x)
- 3% acetic acid solution
3 ml glacial acetic acid
97 ml distilled water
- 2% potassium hydroxide (KOH) solution
2 g potassium hydroxide
100 ml distilled water
Mix well by using the ceramic plate and the magnetic stir bar
- Alcian blue solution (pH 2.5)
1 g Alcian blue, 8GX
100 ml 3% acetic acid solution
Mix well by using the ceramic plate and magnetic stir bar. Adjust pH to 2.5 using acetic acid
- Alizarin red solution
0.5 mg Alizarin red S
100 ml 1% potassium hydroxide
Mix well by using the ceramic plate and magnetic stir bar. Final concentration is 0.005% (w/v) alizarin red in 1% potassium hydroxide
- 1% KOH/20% glycerol solution
20 ml glycerol
50 ml KOH (2%)
30 ml distilled water
Mix well by using the ceramic plate and magnetic stir bar
Acknowledgments
We would like to thank Ali Naji and Dr. Katherine Kin for their excellent help with the ex-vivo mandibular explant assay. The contribution of Victoria Xie who created illustrative Figure 2 is greatly appreciated. This study was supported by the Bone Disease Program of Texas and NIH/R15GM122030-01 to Dr. Walid Fakhouri from the University of Texas Health Science Center, School of Dentistry at Houston. The authors declare no conflicts of interest or competing interests.
References
- Chai, Y., Mah, A., Crohin, C., Groff, S., Bringas, P., Jr., Le, T., Santos, V. and Slavkin, H. C. (1994). Specific transforming growth factor-β subtypes regulate embryonic mouse Meckel's cartilage and tooth development. Dev Biol 162(1): 85-103.
- Fakhouri, W. D., Metwalli, K., Naji, A., Bakhiet, S., Quispe-Salcedo, A., Nitschke, L., Kousa, Y. A. and Schutte, B. C. (2017). Intercellular genetic interaction between Irf6 and Twist1 during craniofacial development. Sci Rep 7(1): 7129.
- Jin, Y. R., Turcotte, T. J., Crocker, A. L., Han, X. H. and Yoon, J. K. (2011). The canonical Wnt signaling activator, R-spondin2, regulates craniofacial patterning and morphogenesis within the branchial arch through ectodermal-mesenchymal interaction. Dev Biol 352(1): 1-13.
- Metwalli, K. A., Do, M. A., Nguyen, K., Mallick, S., Kin, K., Farokhnia, N., Jun, G. and Fakhouri, W. D. (2017). Interferon Regulatory Factor 6 is necessary for salivary gland and pancreas development. J Dent Res 1-11.
- Miettinen, P. J., Chin, J. R., Shum, L., Slavkin, H. C., Shuler, C. F., Derynck, R. and Werb, Z. (1999). Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nature Genetics 22(1): 69-73.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jiang, J., Bertol, J. W. and Fakhouri, W. D. (2017). Mandibular Explant Assay for Investigating Extrinsic Stimuli on Bone and Cartilage Development. Bio-protocol 7(23): e2641. DOI: 10.21769/BioProtoc.2641.
Category
Developmental Biology > Cell growth and fate > Cartilage
Neuroscience > Development > Explant culture
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










