- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Rice Stripe Virus Preparation from Viruliferous Small Brown Planthoppers and Mechanic Inoculation on Rice
Published: Vol 7, Iss 21, Nov 5, 2017 DOI: 10.21769/BioProtoc.2597 Views: 7039
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3038 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2129 Views
Abstract
Tenuiviruses can infect the plants of the family Poaceae, and cause serious loss of crops, particularly rice and maize, in South-Eastern Asian countries. Tenuiviruses usually depend on insect vectors for their transmission and cannot be transmitted between plants through wounds or abrasions. Rice stripe virus (RSV), a typical member of tenuiviruses, is efficiently transmitted by the small brown planthopper Laodelphax striatellus in a persistent-propagative manner to cause rice stripe disease. Here we presented a convenient method, the midrib micro-injection, to mechanically inoculate insect-derived RSV into rice leaves for conducting pathogenicity assay on rice plants.
Keywords: Rice stripe virusBackground
Tenuiviruses cannot be mechanically inoculated into plants, unless through vascular puncture inoculation with quite different transmission rates ranging from 1% to 90% according to different experimental details (Louie, 1995; Hogenhout et al., 2008). As to RSV, mechanical transmission usually fails or yields a low infectious rate (Ling, 1972). In particular, the transmission rate was only 6% after the injection of the RSV crude extraction from diseased plants (Okuyama and Asuyama, 1959). The midrib micro-injection method mentioned in this work promotes the RSV transmission rate to 17%. Though the incidence of RSV by mechanical transmission is still much lower than that by insect vector transmission (53%), our method provides a convenient way for mechanical inoculation of persistent-propagative plant viruses. Moreover, based on this method, replication and gene expression of a persistent-propagative plant virus can be determined more accurately in infected plant hosts without the interference of insects, i.e., the inoculation doses and the insect proteins.
Materials and Reagents
- 15 ml centrifuge tube, RNase-/DNase-free (Corning, catalog number: 430791 )
- 1.5 ml clear Microtubes (Corning, Axygen®, catalog number: MCT-150-C )
- Plastic tissue grinder pestles (Tiangen Biotech, catalog number: OSE-Y001 )
- Pipettes with tips of 10 μl, 100 μl, and 1,000 μl (Eppendorf, catalog numbers: 3120000020 , 3120000046 and 31200000623 )
- PVDF Western blotting membranes (Sigma-Aldrich, catalog number: 03010040001 )
Note: Currently, it is ‘Merck, catalog number: 03010040001 ’. - Extra thick blot paper filter paper (Bio-Rad Laboratories, catalog number: 1703965 )
- 96-well ELISA Microplates (Greiner Bio One International, catalog number: 650001 )
- Adhesive plastic
- 25 G ⅝ to 30 G ½ gauge needle
- Drummond replacement glass capillaries, 100/vial (Drummond Scientific, catalog number: 3-000-203-G/X )
- Parafilm® M (Sigma-Aldrich, catalog number: P7793 )
Note: Currently, it is ‘Merck, catalog number: P7793 ’. - Insect vectors (Laodelphax striatellusi, Fallen) (collected from a field population in Hai’an, Jiangsu Province, China)
- RSV cp gene was amplified with the primers: 5’-ATGGGTACCAACAAGCCAGC-3’, and 5’-CTAGTCATCTGCACCTTCTG-3’. PCR was run on the ProFlexTM PCR System under cycling conditions of 95 °C for 5 min, followed by 30 cycles of 95 °C for 20 sec, 50 °C for 30 sec and 72 °C for 30 sec. The purified 969 bp of PCR products were cloned into pET-28a (Merck, Novagen, catalog number: 69865-3 ) for Cp protein expression. The recombinant cp plasmid was sent to Beijing Genomics Institute for monoclonal anti-Cp antibody production
- Host plants (Oryza sativa L. spp. japonica var. Nippobare)
Note: Healthy 2-week-old rice leaves with the length of approximately 15 cm were used for microinjection. - Protein loading buffer (5x) (Adipogen International, catalog number: AG-10T-0020-L001 )
- ExpressPlusTM PAGE gel, 4-20% (GenScript, catalog number: M42010 )
- PageRulerTM prestained protein ladder, 10 to 180 kDa (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 26616 ), used for SDS-PAGE
- HEPES (Fisher Scientific, catalog number: BP310-100 ), used for SDS-PAGE
- EMPLURA® methanol (Merck, catalog number: 822283 )
- ELISA coating buffer, 1x (Solarbio, catalog number: C1050 )
- SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 34095 )
- Goat anti-mouse IgG (H+L) secondary antibody, HRP (Thermo Fisher Scientific, InvitrogenTM, catalog number: 32430 )
- Anti-Cp antibody (Beijing Genomics Institute)
- 3,3’,5,5’-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA (Sigma-Aldrich, catalog number: T0440 )
Note: Currently, it is ‘Merck, catalog number: T0440 ’. - Sulfuric acid (H2SO4) (5 N) (Merck, catalog number: 480364 ), used for ELISA
- Sodium dodecyl sulfate (SDS) (AMRESCO, catalog number: 0227 ), used for ELISA
- Mineral oil (Sigma-Aldrich, catalog number: M8410 )
Note: Currently, it is ‘Merck, catalog number: M8410 ’ . - Tween® 20 (Sigma-Aldrich, catalog number: P1379 )
- Phosphate buffer saline (PBS), pH 7.4, basic (1x) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010031 )
- OxoidTM Skim milk power (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: LP0031B )
- Phosphate buffer saline with Tween 20 (1x) (PBST) (see Recipes)
- Transfer buffer (see Recipes)
- Blocking buffer (see Recipes)
- Diluted monoclonal anti-Cp antibody (see Recipes)
- Diluted Goat anti-mouse IgG, HRP-conjugated antibody (see Recipes)
Equipment
- TGrinder High Speed Tissue grinder (Tiangen Biotech, catalog number: OSE-Y30 )
- Centrifuge 5424R (Eppendorf, model: 5424 R )
- Decolorizing Orbital Shaker (T-Bota Scietech Instruments & Equipment, model: TS-200 ), used for antibody incubation in ELISA and Western blots
- Chemiluminescent Imaging System (Tanon, model: Tanon 5200 )
- SpectraMax® Paradigm® Multi-Mode Detection Platform (Molecular Devices, model: SpectraMax Paradigm Multi-Mode )
- Micropipette Puller (Sutter Instrument, model: P-97 )
- ProFlexTM PCR System (Thermo Fisher Scientific, Applied BiosystemsTM, model: ProFlexTM 3 x 32-Well PCR System, catalog number: 4484073 )
- Glass incubators (Φ55 x 150 mm, Φ110 x 150 mm), used for planthopper incubation
Note: Each glass incubator must be sealed with a nylon mesh in order to protect the cultured plants or insects from other interference sources. The culture condition is 25 °C, with 16 h of light daily (Figure 1).
Figure 1. Plant culture and insect rearing. The small brown planthoppers and rice seedlings are cultured in glass incubators sealed with nylon meshes. The viruliferous or nonviruliferous planthoppers are geographically isolated, which are cultured in different insectaries. - MixMate® , the 3-in-1 mixer (Eppendorf, catalog number: 5353000014 )
- H2O3 dry bath (Coyote Bioscience, model: H2O3-100C )
- PowerPacTM universal power supply (Bio-Rad Laboratories, catalog number: 1645070 )
- Trans-Blot® TurboTM transfer system (Bio-Rad Laboratories, catalog number: 1704150 )
- Nanoject II Programmable Nanoliter injector (World Precision Instruments, model: Nanoliter 2000 )
- Backfilling Needle for Nanoject II Auto-Nanoliter Injector, 2” Length (Drummond Scientific, model: 3-000-027 )
- SZ61 Stereo microscope (Olympus, model: SZ61 ), used for microinjection
Procedure
- Preparation of RSV crude extractions
- For RSV extraction, use 100-200 whole bodies of viruliferous small brown planthoppers as samples.
Note: Insects should be directly grinded upon collection. - Brush down the viruliferous planthoppers into a 15 ml tube, and put it on ice for 2-3 min in order to freeze them.
- Fully grind total planthoppers in 800 μl of PBS-buffer (pH 7.2) (v:v, 1:1) with TGrinder, and maintain the tube in ice.
Note: No research has been reported how to extract plant viruses from insect vectors, whereas several researches suggested that RSV-infected plants should be extracted in PBS buffer at 4 °C (Ishikawa et al., 1982; Ishikawa et al., 1989). So in this work, we grind the planthoppers at the same condition. - Shake the mixture thoroughly until well blended, and then place the tube in ice for 30 min.
- Centrifuge the mixture at 1,344 x g for 5 min at 4 °C, and transfer the supernatant into a 1.5 ml pre-cooling RNA-free tube.
- Repeat step A5.
- Centrifuge at 8,400 x g for 15 min at 4 °C, and store the supernatant at 4 °C for further usage.
Note: The supernatant is RSV crude extraction. - Use the same protocol to isolate the crude extractions from nonviruliferous small brown planthoppers as negative controls.
Note: Both RSV crude extractions and the extractions from nonviruliferous planthoppers that used for mechanical inoculation should be microinjected into rice leaves as soon as possible.
- For RSV extraction, use 100-200 whole bodies of viruliferous small brown planthoppers as samples.
- Detection of RSV through Western blots
- For RSV detection, mix 20 µl 5x protein loading buffer with 80 µl extractions from both viruliferous and nonviruliferous planthoppers, and boil the mixture for 5 min.
- Load 20 µl of the treated samples into each well of the ExpressPlus TMPAGE gel, along with prestained marker.
Note: Make sure that the treated samples from viruliferous and nonviruliferous planthoppers will not become cross-contamination. - Run the gel for 40 min at 140 V.
- Prepare a PVDF membrane which was slightly bigger than the size of the gel.
- Place the PVDF membrane in methanol for 5 min.
- Place the PVDF membrane and gel in 1x transfer buffer (see Recipes) for 10 min.
- Prepare the transfer sandwich and place it in a transfer tank with 25 mA for 30 min.
Note: The transfer sandwich includes a layer of filter paper at each end, the PVDF membrane, and gel. The PVDF membrane is placed between the gel surface and the positive electrode in the sandwich. - Block the membrane in 5% skimmed milk in TBST at room temperature (RT) for 0.5-1 h.
- Wash the membrane with PBST (see Recipes) three times for 5 min each time.
- Incubate the membrane in the monoclonal anti-Cp antibody solution (see Recipes) by using Decolorizing Orbital Shaker at RT for 1.5 h or at 4 °C overnight.
- Repeat step B9.
- Incubate in the HRP-conjugated antibody solution by using Decolorizing Orbital Shaker at RT for 1.5 h.
- Wash the membrane with PBST five times for 5 min each time.
- Use the West Femto Substrate Trial Kit to the blot according to the manufacturer’s instruction.
Note: Prepare working solution by mixing 100 µl of the stable peroxide solution and the luminol/enhancer solution. Use 100 µl working solution per cm2 of membrane. - Capture the chemiluminescent signals through Chemiluminescent Imaging System.
Note: Cp could be only detected from the extractions of the viruliferous small brown planthoppers (Figure 2).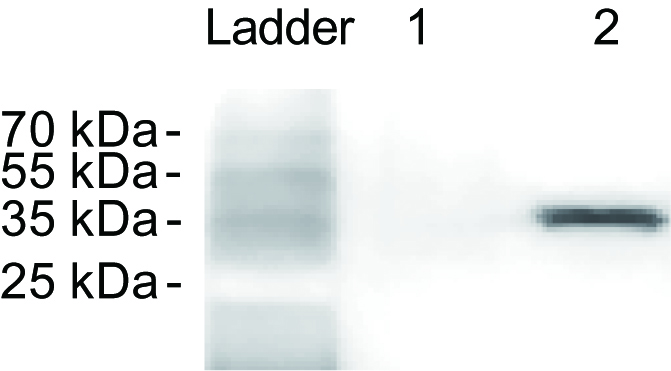
Figure 2. RSV detection of the crude extractions from nonviruliferous (lane 1) and viruliferous (lane 2) small brown planthoppers. The monoclonal anti-Cp antibody was used as the primary antibody.
- For RSV detection, mix 20 µl 5x protein loading buffer with 80 µl extractions from both viruliferous and nonviruliferous planthoppers, and boil the mixture for 5 min.
- Quantification of RSV crude extractions
- Prepare the Cp standards in PBS-buffer (pH 7.2) according to previously optimized standard ELISA protocol (Nemzek et al., 2001). Nine dilutions are made (0, 0.0045, 0.045, 0.45, 4.5, 12.5, 25, 45, 90 ng/ml).
- Use RSV crude extractions as samples.
Note: Samples for ELISA should be tested in duplicate. - Add 100 µl of the standards and samples into each well of a 96-well ELISA plate, and mix them with 100 µl 1x coating buffer. Cover the plate with an adhesive plastic and incubate at 4 °C overnight.
- Wash the plate three times by filling each well with 200 µl PBST, at least for 3 min each time.
- Block the remaining protein-binding sites in the wells by adding 200 µl blocking buffer (see Recipes) per well. Cover the plate with an adhesive plastic and incubate at RT for no less than 30 min.
- Repeat step C4.
- Add 100 µl of diluted monoclonal anti-Cp antibody to each well. Cover the plate with an adhesive plastic and incubate at RT for 1.5 h.
- Repeat step C4.
- Add 100 µl of diluted Goat anti-mouse IgG, HRP-conjugated antibody to each well (see Recipes). Cover the plate with an adhesive plastic and incubate at RT for 1.5 h.
- Remove the supernatant and wash the plate five times with PBST, for 1 min each time.
- Dispense 100 µl of TMB solution into each well.
- Gently mix on Decolorizing Orbital Shaker at RT for 30 min.
Note: This reaction should be protected from light. - Add 100 µl of 2 M H2SO4 or 1% SDS to stop the reaction.
Note: Make sure that the color has changed from blue to yellow in all the wells. - Read the optical density (OD) at 450 nm on the SpectraMax® Paradigm® Multi-Mode Detection Platform within 5 min.
Note: You can also read the absorbance value of OD630 after chromogenic reaction without adding stop solution.
- Prepare the Cp standards in PBS-buffer (pH 7.2) according to previously optimized standard ELISA protocol (Nemzek et al., 2001). Nine dilutions are made (0, 0.0045, 0.045, 0.45, 4.5, 12.5, 25, 45, 90 ng/ml).
- Microinjection of RSV into rice leaves
- Prepare the needles for microinjection on a Micropipette Puller (Dean, 2006) (Video 1).
Note: Needles for microinjection are pulled from glass capillaries, and 25 G ⅝ to 30 G ½ gauge needle is ideal for injection.Video 1. Preparing the needles for microinjection on the Micropipette Puller - Fill the glass capillary with mineral oil before installation.
- Dilute the RSV crude extraction with PBS buffer to a concentration of Cp around 10 ng/ml.
- Empty the needle and fill with the diluted RSV crude extraction.
Note: The diluted RSV crude extraction should be placed on a Parafilm and then be inhaled into the needle. - Microinject 23 nl of the diluted RSV crude extraction into the midribs of the healthy 2-week-old rice leaves for five times through a glass needle at slow speed using Nanoliter 2000. The interval between each injection site is 1 cm (Video 2). Both sides of the leaves were OK for microinjection.
Note: The midrib should be observed to be filled with RSV crude extraction upon microinjection (Figure 3).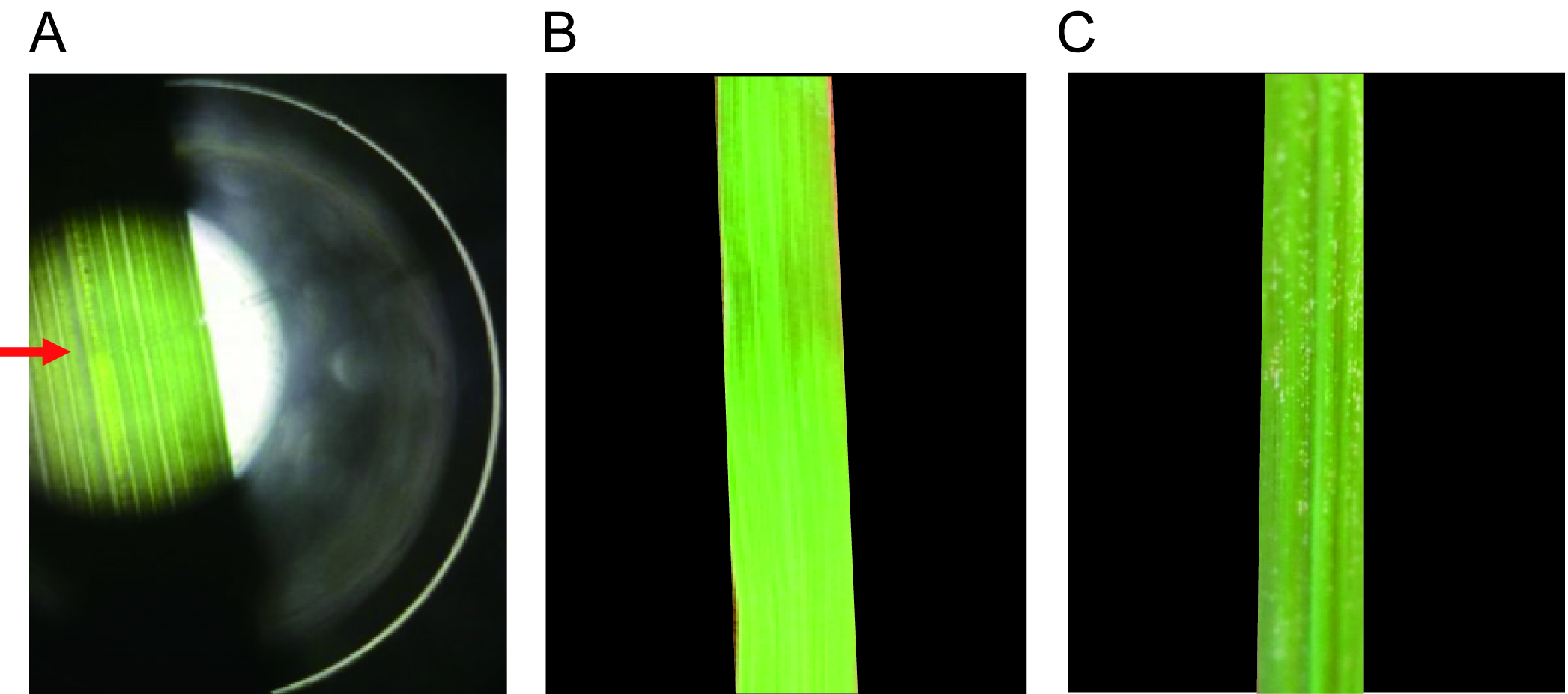
Figure 3. RSV microinjection and symptom observation. A. RSV microinjection. RSV crude extractions were microinjected into the midribs of rice leaves through Nanoliter 2000. The red arrow indicates the injection site. Symptoms of rice leaves inoculated with extractions from nonviruliferous planthoppers (B) and from viruliferous planthoppers (C) were observed two weeks later. The RSV extractions from viruliferous planthoppers induced typical disease symptoms.Video 2. Microinjection of RSV through Nanoject II Programmable Nanoliter injector - Microinject the extractions from nonviruliferous small brown planthoppers into the midribs of healthy rice leaves as negative control.
Note: The negative control is very important as it can tell you whether or not the experimental system works. - Evaluate the development of disease symptoms of both the injected leaves and systemic leaves after about 2-3 weeks post inoculation. The disease symptoms are evaluated as described by Toriyama and Sakurai (1969).
Note: Make at least three replicates, and each group contains at least 30 seedlings. The incidence of RSV by mechanical transmission is about 17% after the midrib microinjection of insect-derived RSV crude extractions.
- Prepare the needles for microinjection on a Micropipette Puller (Dean, 2006) (Video 1).
Data analysis
Calculation of ELISA results
- Calculate the mean of OD630 or OD450 values for each group of standards and samples.
Note: In this experiment, OD630 was measured after chromogenic reaction without adding stop solution. - Build a standard curve by plotting the mean OD value obtained from each standard against its concentration in ng/ml on linear graph paper. X-axis shows the concentrations of standard proteins, and Y-axis shows the corresponding absorbance values (Figure 4).
- Determine the corresponding concentration of each sample in ng/ml by using the mean OD values based on the standard curve.
- Multiply the derived concentrations by the dilution factor to determine the actual concentration of Cp protein in the RSV crude extraction samples.
Note: Make sure that samples are diluted appropriately, the OD values of which are within the scope of the standard curve.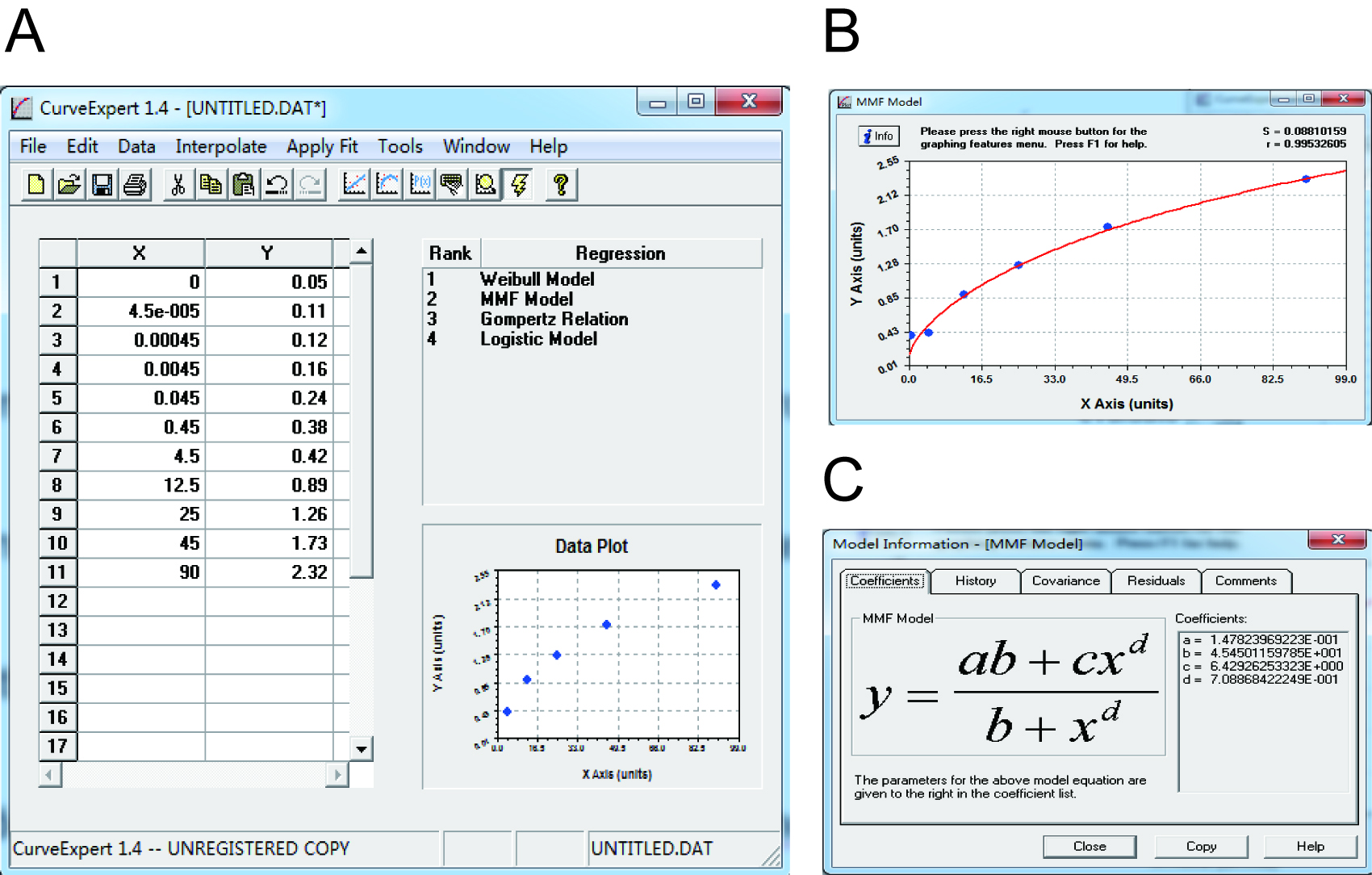
Figure 4. Data analysis of ELISA result by using CurveExpert 1.4. A. Data input. The concentrations of Cp and the OD630 values were listed as the X and Y axis. B. Standard curve construction. The standard curve was built based on data plot. S and r value were listed in the top right corner. C. The equation of the standard curve. The equation was automatically calculated. The parameters were listed in the right pane.
Notes
- It is recommended that during the ELISA and microinjection step, extractions from healthy insect samples should be used as negative controls, in order to evaluate the experimental systems.
- RSV crude extractions from nonviruliferous or viruliferous planthoppers are stored at 4 °C for no longer than two days. Generally, the RSV crude extractions with the OD value above 1.00 (Cp concentration around 10 ng/ml) is suitable for microinjection.
Recipes
- Phosphate buffer saline with Tween 20 (1x) (PBST)
0.2% Tween 20
Add to PBS buffer (pH 7.2)
Filter sterilize using filtering device - Transfer buffer (semi-dry)
48 mM Tris
39 mM glycine
20% methanol
0.04% SDS - Blocking buffer
1 or 5% skim milk
Add to TBST buffer
Mix well and filter
Store at 4 °C for no longer than 3 days - Diluted monoclonal anti-Cp antibody
Add 5 μl monoclonal anti-Cp antibody into 10 ml blocking buffer (5% skim milk)
Store at 4 °C for no longer than 1 day - Diluted Goat anti-mouse IgG, HRP-conjugated antibody
Add 2 μl monoclonal anti-Cp antibody into 10 ml blocking buffer (5% skim milk)
Store at 4 °C for no longer than 1 day
Acknowledgments
This work is adapted from previously published papers (Zhao et al., 2016). We acknowledge the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB11040200) and the Major State Basic Research Development Program of China (973 Program) (No. 2014CB13840402) for funds. The authors declare no conflict of interests.
References
- Dean, D. A. (2006). Preparation (pulling) of needles for gene delivery by microinjection. CSH Protoc 7.
- Hogenhout, S. A., Ammar el, D., Whitfield, A. E. and Redinbaugh, M. G. (2008). Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46: 327-359.
- Ishikawa, K., Omura, T. and Hibino, H. (1982). Characterization of rice stripe virus a heavy component carrying infectivity. J Gen Virol 61(2): 187-195.
- Ishikawa, K., Omura, T. and Tsuchizaki, T. (1989). Association of double-and single-stranded RNAs with each four components of rice stripe virus. Jan J Phytopathol 55: 315-323.
- Ling, K. C. (1972). Rice virus diseases. 148.
- Louie, R. U. (1995). Vascular puncture of maize kernels for the mechanical transmission of maize white line mosaic virus and other viruses of maize. v.85. (ARS, Corn and Soybean Research Unit, Wooster).
- Nemzek, J. A., Siddiqui, J. and Remick, D. G. (2001). Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods 255(1-2): 149-157.
- Okuyama, S. and Asuyama, H. (1959). Mechanical transmission of rice stripe virus to rice plant. Ann PSJ 24: 35.
- Toriyama, K. and Sakurai, Y. (1969). Breeding rice varieties for resistance to stripe virus disease. Jpn Agr Res Q 4: 5.
- Zhao, W., Yang, P., Kang, L. and Cui, F. (2016). Different pathogenicities of Rice stripe virus from the insect vector and from viruliferous plants. New Phytol 210(1): 196-207.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhao, W., Kang, L. and Cui, F. (2017). Isolation of Rice Stripe Virus Preparation from Viruliferous Small Brown Planthoppers and Mechanic Inoculation on Rice. Bio-protocol 7(21): e2597. DOI: 10.21769/BioProtoc.2597.
Category
Microbiology > Microbe-host interactions > Virus
Plant Science > Plant physiology > Phenotyping
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











