- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessment of Aversion of Acute Pain Stimulus through Conditioned Place Aversion
Published: Vol 7, Iss 21, Nov 5, 2017 DOI: 10.21769/BioProtoc.2595 Views: 8100
Reviewed by: Geoff LauJuan Facundo Rodriguez AyalaQing Yan

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evaluating Working Memory on a T-maze in Male Rats
Ahmed M. Hussein [...] Volker Korz
Jul 20, 2018 10280 Views

Classic Labyrinth Test for Neurobehavioral Evaluation in Wistar Rats
Salim Gasmi
Sep 20, 2018 6880 Views

Consummatory Successive Negative Contrast in Rats
Ana María Jiménez-García [...] Ignacio Morón
Apr 5, 2019 5054 Views
Abstract
Pain is a complex experience. The aversive component of pain has been assessed through conditioned place aversion in rodents. However, this behavioral test does not allow the evaluation of the aversion of an acute pain stimulus. In Zhang et al. (2017), we provide an updated version of a Conditioned Place Aversion paradigm to address this challenge. In this protocol, a detailed version of this method is described.
Keywords: Conditioned Place AversionBackground
Pain is a multidimensional experience that includes sensory and affective components. As such, behavioral tests that assess both the sensory and the emotional aspects of pain are critical to understanding the whole pain experience. However, the majority of available behavioral tests depends on the measurement of nociceptive stimuli and relies on withdrawal thresholds, which are reflexive responses. Nociceptive withdrawal reflex behaviors are under supraspinal control and can occur in the absence of supraspinal inputs. On the other hand, higher order pain behaviors such as conditioned place aversion (CPA) assess some of the emotional component of pain. These experiments involve having the animal choosing to avoid or escape a pain-inducing behavior and/or treatments by moving to the other chamber of the box (LaBuda and Fuchs 2000; Ding et al., 2005; van der Kam et al., 2008; McNabb et al., 2012; He et al., 2012). Lesions of the anterior cingulate cortex (ACC) block the development of escape-avoidance pain behaviors (Johansen et al., 2001; LaGraize et al., 2004; Qu et al., 2011). However, those previous behavioral assays always implied an avoidance learning paradigm, and the conditioning lasts for consecutive days. These assays also require that pain stimulation be applied for prolonged periods of time during the multi-day conditioning phases, and thus most of these assays employed a persistent or chronic pain model. As a result, these assays do not assess the aversive response to an acute pain stimulus. Therefore, testing the aversion of acute pain stimulus is urgently needed to allow for the accurate and comprehensive assessment of acute pain and the screening of analgesics.
With this in mind, we modified the previously described conditioned place aversion test. We applied an acute nociceptive stimulus to the hind paw of the animals. This stimulus was short-lived, as a paw withdrawal removed it. To make sure of a behavioral response, we repeated this stimulus. However, to ensure that we are testing an acute pain response, we shortened the experiment to 3 consecutive sessions of 10 min, with 10 min total for the pain conditioning phase.
Through this assay, we can observe an avoidance behavior which is short lasting and easily regulated by optogenetic modulation of ACC neurons.
Materials and Reagents
- Male Sprague-Dawley rats, 250-300 g (Taconic Biosciences, catalog number: SD-M )
- 70% ethanol solution (Decon Labs, catalog number: 2716 )
- Nivea Lip Care A Kiss of Cherry Fruity Lip Care 0.17 oz. (Walgreen, Nivea, catalog number: 331781 )
- Nivea Lip Care A Kiss of Mint & Minerals Refreshing Lip Care 0.17 oz. (Walgreen, Nivea, catalog number: 443307 )
- Isoflurane (Piramal Critical care, NDC 66794-017-25)
- AAV1.CAMKII.ChR2-eYFP.WPRE.hGH (Penn vector Core, University of Pennsylvania)
- AAV1.CAMKII.NpHR-eYFP.WPRE.hGH (Penn vector Core, University of Pennsylvania)
Equipment
- Hamilton syringe, 75 ASN, 26 G, 5 µl (Hamilton, catalog number: 87989 )
- Two High Power blue DPSS Lasers HPL1 and HPL2, 1,000 mW, 473 nm (Shanghai Dreams Laser Technology, catalog number: SDL-473-1000T )
- Low Power blue DPSS Laser LPL, 200 mW, 473 nm (Shanghai Dream Lasers Technology, catalog number: SDL-473-10T )
- Low Power yellow DPSS Laser LPL, 50 mW, 589 nm (Ultralasers, model: MGL-III-589-50 )
- Digital Optical Power and Energy Meter (Thorlabs, model: PM100D )
- Standard Photodiode Power Sensor (Thorlabs, model: S121C )
- Mesh table (IITC Life Science, catalog number: 410 )
- USB 3.0 monochrome industrial camera (The Imaging Source, catalog number: DMK 23U618 )
- Varifocal manual iris lens (Computar, catalog number: T3Z3510CS )
- 1x2 fiber-optic rotary joint (Doric Lenses, model: FRJ_1x2i_FC-2FC )
- TTL pulse generator (Doric Lenses, model: OTPG_4 )
- Dohm sound machine (Marpac, catalog number: Marpac Dohm DS )
- Standard desktop computer or laptop
- Metal stand (Humboldt, catalog number: H-21330 )
- 2 chambered apparatus (designed in the laboratory, 16 x 7 x 13 cm) made from black (color number 2025) opaque acrylic, 1/4” (5.6 mm) thick (Canal Plastics Center, NY, USA)
- Mini Glue Gun, 15 Watt, 1/4 In (Stanley Black & Decker, model: GR10 , catalog number: 3NZW9Mfr)
- Clear Hot Melt Glue Stick, 1/4” Diameter, 4” Length, 24 PK (Stanley Black & Decker, model: GS10DT , catalog number: 2FDB9 Mfr.)
- Ceramic LC MM Ferrule 1.25 mm (Thorlabs, catalog number: CFLC230-10 )
- Ceramic split mating sleeve (Thorlabs, catalog number: ADAL1 )
- Fiber cable (Thorlabs, catalog number: M83L01 )
- Dental Cement Unifast Trad powder (Pearson Dental Supply, catalog number: G 05-12-24 ) and liquid (Pearson Dental Supply, catalog number: G 05-12-26 )
Software
- ANY-maze tracking software (Stoelting Co., Illinois, USA, www.stoeltingco.com)
- GraphPad Prism version 7 (GraphPad Software Inc, California, USA, www.graphpad.com)
- OTPG4 software (Doric Lenses Inc, Quebec, Canada, http://doriclenses.com)
Procedure
For the outline of this protocol, see Figure 1.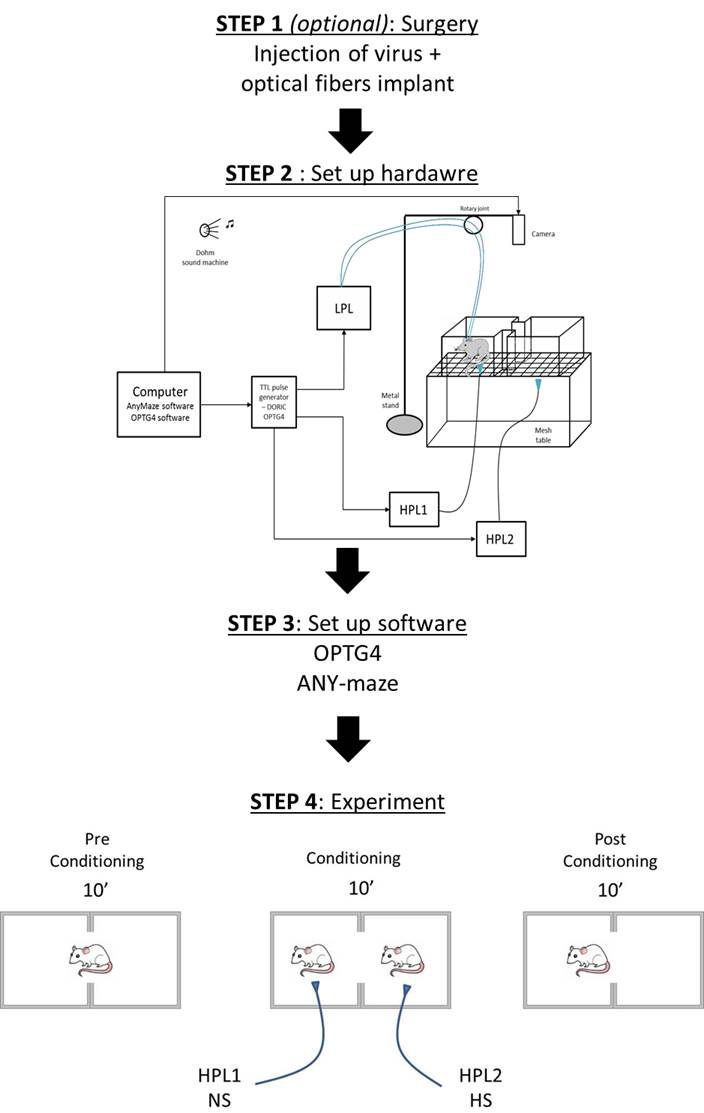
Figure 1. Flowchart of the basic steps of the protocol
- Hardware setup (detailed Figure 2)
For these experiments, we used thermal stimuli applied via High Power Lasers (HPL), and modulate the neuronal activity using optogenetic stimulation. The set up described above, can be customized to fit another type of stimulus.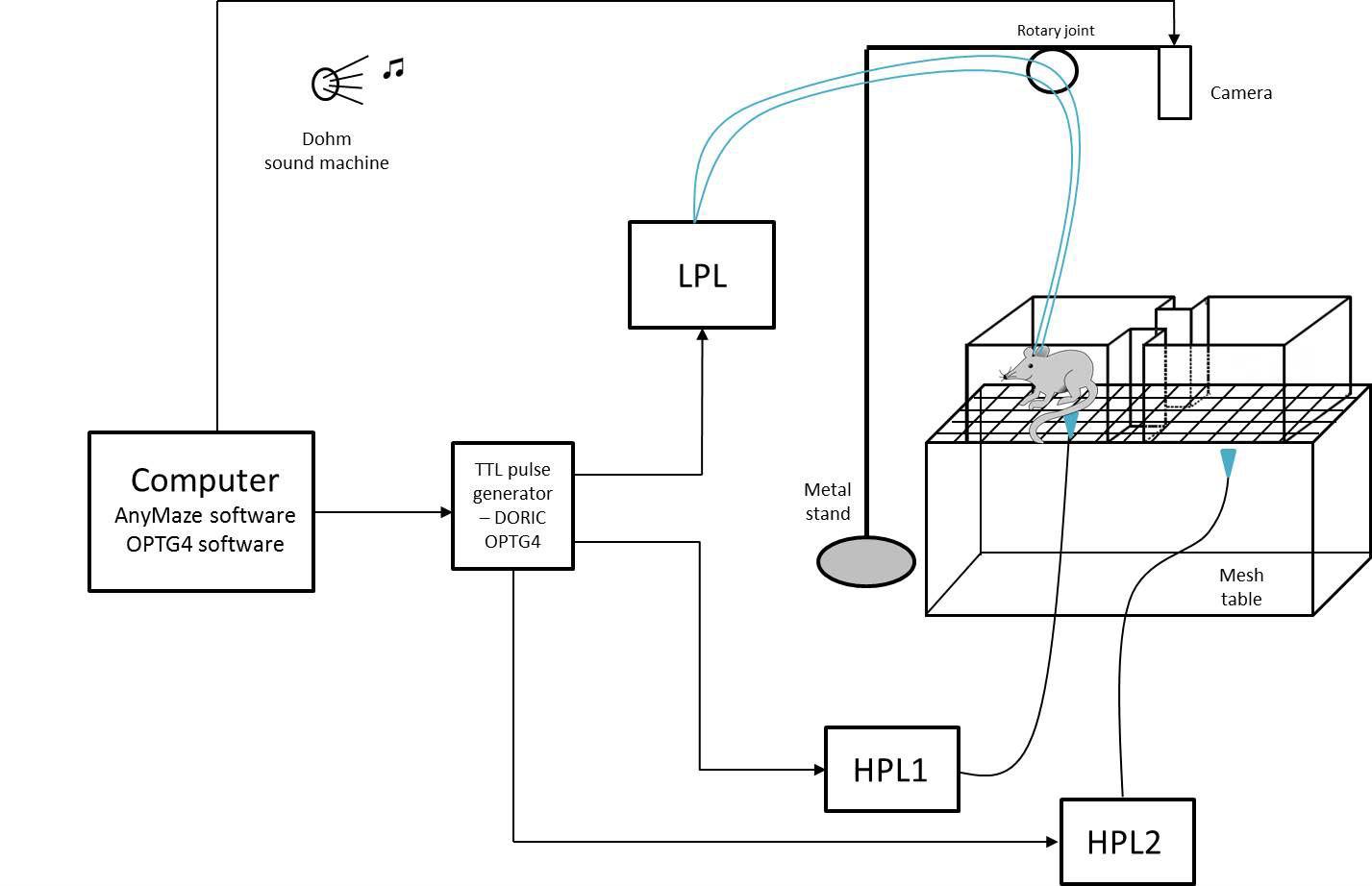
Figure 2. Schematic of the complete set up used for performing a Conditioned Place Aversion experiment. CPA apparatus is placed on the mesh table. Animals are recorded via a camera connected to ANY-maze software. A High Power Laser (HPL) delivers a thermal stimulus applied by the experimenter, and a Low Power Laser (LPL) delivers optogenetic light stimulation. The LPL connects 2 fiber cables to the 2 optical fibers implanted in the head of the animal for bilateral optogenetic stimulation of the ACC. All lasers are controlled via a TTL pulse generator in the OPTG4 software to deliver the light stimuli synchronically.- We used a modified escape-avoidance paradigm as previously published (LaBuda and Fuchs, 2000). The apparatus was made of Plexiglas with dimensions 16 x 7 x 13 cm and placed on top of a mesh table (Figure 3). The box was divided into two chambers. To allow the animal to discriminate between the 2 chambers, each one is paired with different cues (any kind of smell, visual, or tactile cues). In our experiment, we applied different smell cues inside each lateral side of the 2 chambers (right chamber = cherry fruity smell/left chamber = mint smell).
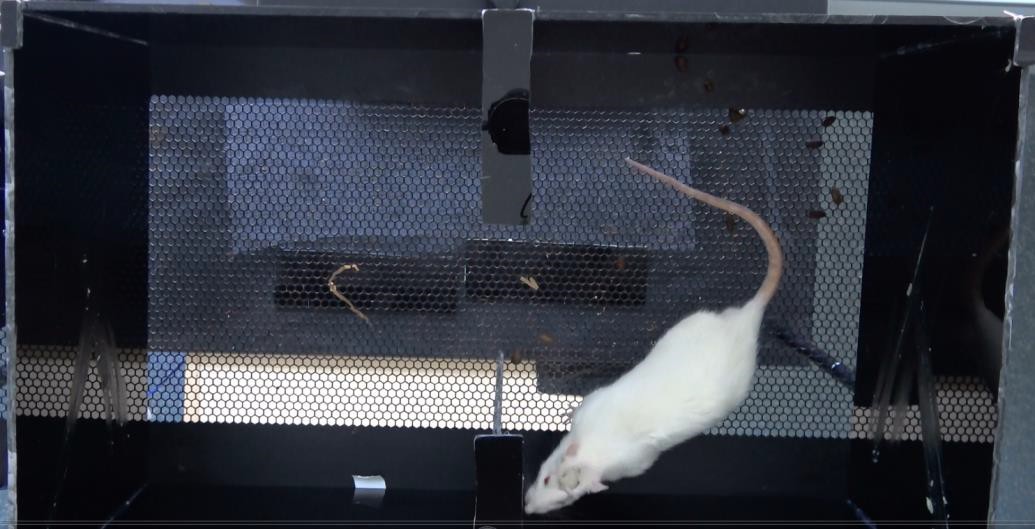
Figure 3. Rat in the CPA apparatus. The rat is allowed to freely move between the two chambers during the 3 phases of the experiment. Its movements are recorded by the camera linked to the ANY-maze software, which calculates the time spent in each chamber. - Install the camera on the Metal stand above the 2 chambered apparatus.
- Connect the lasers to the TTL pulse generator.
- Connect the TTL pulse generator to the computer.
- Turn on the Dohm sound machine.
- Turn On HPLs and LPL. Let them warm up for 15 min.
- Set HPLs to the desired power using the power meter
HPL1 Non noxious Stimulus NS-50 mW, and HPL2 to High noxious Stimulus HS-250 mW;
HPL1 Low noxious Stimulus LS-150 mW and HPL2 to High noxious Stimulus HS-250 mW;
HPL1 Non noxious Stimulus NS-50 mW and HPL2 to Non noxious Stimulus NS-50 mW;
HPL1 Low noxious Stimulus LS-150 mW and HPL2 to Low noxious Stimulus LS-150 mW;
HPL1 High noxious Stimulus HS-250 mW and HPL2 to High noxious Stimulus HS-250 mW. - Set LPL to the desired power using the power meter for optogenetic stimulation (power = 10 mW).
- We used a modified escape-avoidance paradigm as previously published (LaBuda and Fuchs, 2000). The apparatus was made of Plexiglas with dimensions 16 x 7 x 13 cm and placed on top of a mesh table (Figure 3). The box was divided into two chambers. To allow the animal to discriminate between the 2 chambers, each one is paired with different cues (any kind of smell, visual, or tactile cues). In our experiment, we applied different smell cues inside each lateral side of the 2 chambers (right chamber = cherry fruity smell/left chamber = mint smell).
- Software set up
Ensure that the number of the channel corresponds to the correct port on the TTL pulse generator. HPL1 connected to port entry #1 will be controlled in Channel 1, HPL2 connected to port entry #2 will be controlled in Channel 2, and LPL connected to port entry #3 will be controlled in Channel 3 (Figure 4).
Set the parameters in OTPG4 software to control the HPL.
For optogenetic modulation of neuronal activity:- Set the parameters in OTPG4 software to control the LPL (example: frequency = 20 Hz; Time ON duration = 10 msec, Number of Pulses = 100, Channel 3 in Figure 4).
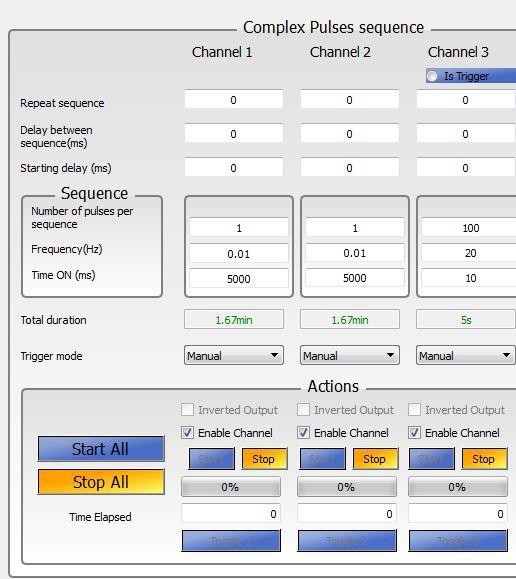
Figure 4. OTPG4 software interface - Turn On the ANY-maze software on the computer
- Start by opening a New Experiment and name it.
- Create a New Protocol (Figure 5).
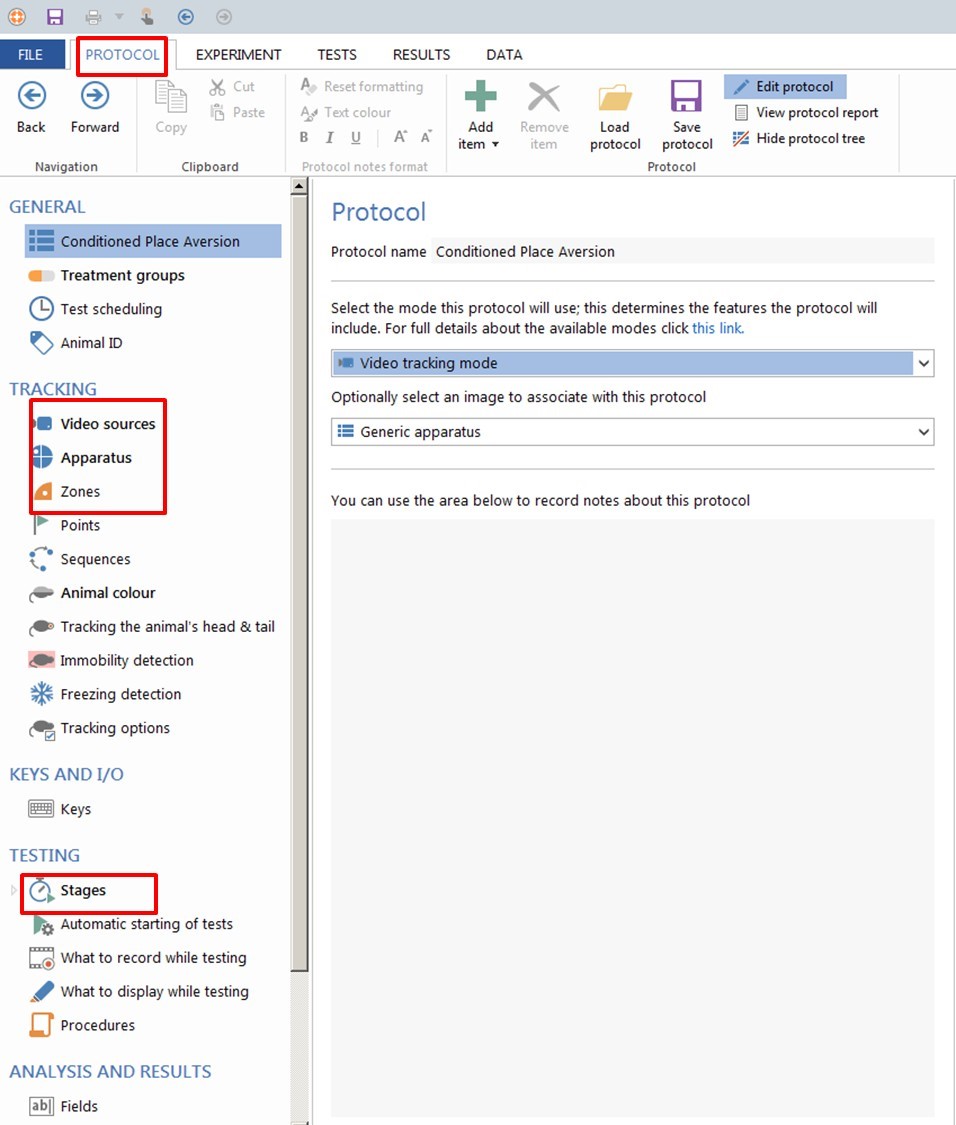
Figure 5. ANY-maze software. How to create a new protocol. - Select the video source corresponding to your camera and set up your image. Name a new apparatus (example CPA).
- Create two zones on the screen (Figure 6), name them and select the settings you want to record during the experiment (time in the zone).
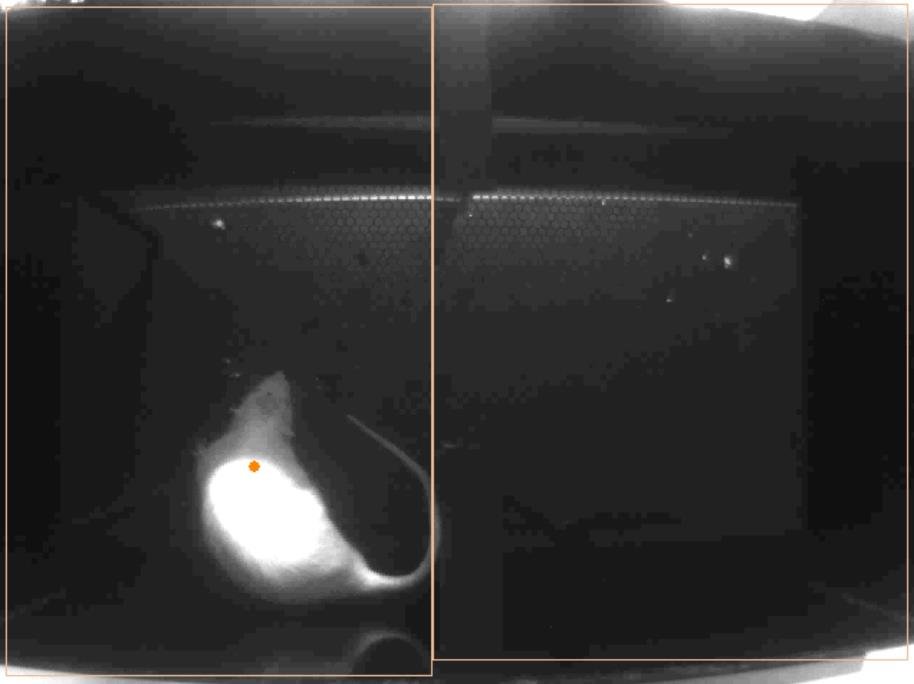
Figure 6. ANY-maze software. How to delimitate 2 zones. - On the stages tab, select the duration of your experiment (600 sec = 10 min in our case) Select the type of data you want to collect in Results, Report and Data.
- Set up the parameters of your Experiment (number of animals, different phases. Figure 7).
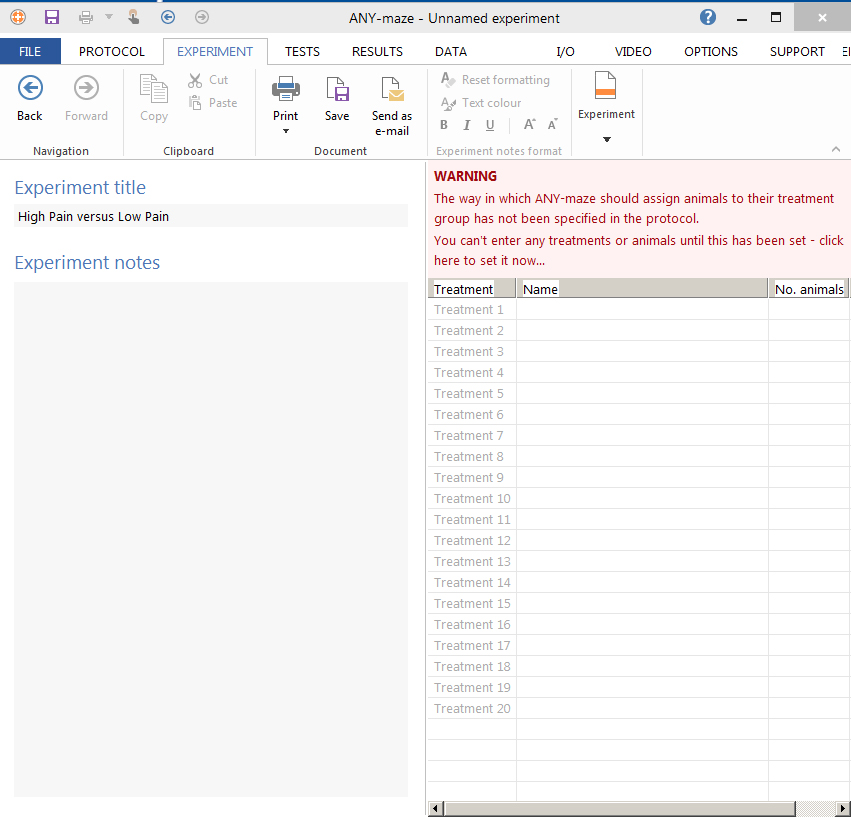
Figure 7. ANY-maze software. Set up for a new experiment. - To start recording, go to the Test section → click on video recording → ‘Play’ button (double click until the chronometer starts).
Note: For any additional parameters, please refer to ANY-maze software Guideline.
- Start by opening a New Experiment and name it.
- Set the parameters in OTPG4 software to control the LPL (example: frequency = 20 Hz; Time ON duration = 10 msec, Number of Pulses = 100, Channel 3 in Figure 4).
- Experiments
- For optogenetic manipulation of neuronal activity, prior to conducting the behavioral experiments, perform transcranial injections of optogenetic constructs. Anesthetize animals with isoflurane (1.5 to 2%). In all experiments, the virus is delivered to the anterior cingulate cortex (ACC) only. Rats are bilaterally injected with 0.5 μl of viral vectors at a rate of 0.1 μl/10 sec with a 26-G 5 μl Hamilton syringe at anteroposterior (AP) +2.6 mm, mediolateral (ML) ±1.6 mm, and dorsoventral (DV) -2.25 mm, with tips angled 28° toward the midline. The microinjection needles are left in place for 10 min, raised 1 mm and left for another minute to allow for diffusion of virus particles away from injection site, while minimizing spread of viral particles along the injection tract.
Rats are then implanted with 200 μm optic fibers held in 2.5 mm ferrules in the ACC: AP +2.6 mm, ML ±1.6 mm, DV -1.25 mm. Fibers with ferrules are held in place by dental acrylic. Allow the virus to be properly expressed in the neurons (2 to 4 weeks depending of the viral vector serotype). - For 1 week prior to beginning experiments, rats are habituated to the experimenter and the environment. Rats must be comfortable with the experimenter to give the best possible results. Rats are handled by the experimenter. If they seemed stressed or anxious, they are put back in their cages and resumed habituation the following day. During all the behavioral phases, animals are allowed to move unrestricted to either side of the box. The movements of rats in each chamber are automatically recorded by a camera and analyzed with the ANY-maze software (Stoelting).
- During the preconditioning phase (Video 1), place rats randomly on either the left or the right side of the box to start. An equal number of animals in each group starts on either the left or the right side of the box. All animals are allowed to explore the two chamber apparatus without restraint for 10 min. The movement of each rat is recorded and analyzed to verify the absence of any preconditioning chamber preference (Figure 9). Animals spending more than 500 sec or less than 100 sec of the total time in any chamber should be eliminated from further testing or analysis (< 20% of total animals).Video 1. Preconditioning phase. All animals are allowed to explore the two chamber apparatus for 10 min. The two chambers are delimited by an orange rectangle and the position of the animal is monitored by a red dot tracking the center of the animal’s body. Time spent in each chamber is recorded by the camera linked to the ANY-maze software.
- Following the preconditioning phase, the rats undergo conditioning for 10 min (Video 2): each chamber is paired with a stimulus (noxious or not, different noxious intensity). As an example, chamber A is paired with a thermal painful stimulus (laser-High Stimulus HS) and the other chamber (B) is paired with a thermal non-painful stimulus (laser-Non Noxious Stimulus NS). Stimuli are given to the hind-paw every 10 sec when the animal is immobile. For each stimulus applied to the rat’s hind paw, bring the laser tip closely to the animal (0.3-0.5 mm without touching the rodent’s skin), between the holes in the grid of the mesh table. Even if the animal is freely moving, it has some immobile periods during which it’s possible for the experimenter to apply the stimulus on a precise location, its hind paw. Paw withdrawal removes the stimulus as the experimenter would stop applying the stimulus to the rodent’s hind paw.Video 2. Conditioning phase. Each chamber is paired with a stimulus. The left chamber is paired with a thermal painful stimulus (laser-High Stimulus HS) and the right chamber is paired with a thermal non-painful stimulus (laser-Non Noxious Stimulus NS). Stimuli are given to the hind-paw every 10 sec. Peripheral stimuli are applied to the hind paws through the mesh table.
As you can observe on this picture (Figure 8), the tip of the laser (red arrow) is positioned between the holes of the mesh table, allowing to come as close as 0.3-0.5 mm to the rat’s hindpaw, standing above.
Note: In some experiments, the thermal stimulus delivered by the HPL is paired with an optogenetic activation or inhibition of ACC neurons controlled by the LPL. If channel 1 controls HPL1 and channel 3 controls the LPL for the optogenetic stimulation you just have to click on ‘Start All’ on the OTPG4 software interface (Figure 4) and you will activate both lasers simultaneously.
Figure 8. How to apply the stimulus - Finally, the animals undergo a test phase (Video 3), where they are allowed to explore the two chamber apparatus for 10 min (Figure 9).Video 3. Test phase. All animals are allowed to explore the two chamber apparatus for 10 min. This phase is comparable to the preconditioning phase and aims to see the effect of the conditioning through a change of time spent in each chamber.
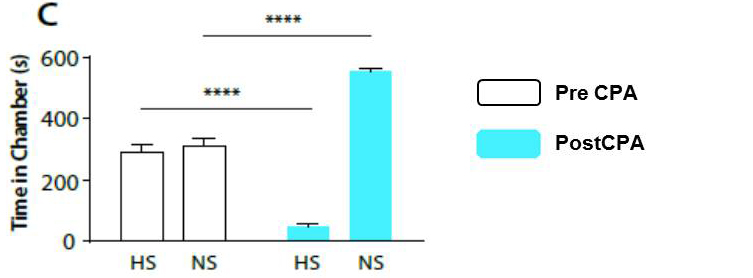
Figure 9. Representative results of CPA behavior (original Figure 1.C [Zhang et al., 2017]: Rats recognize and seek to avoid the aversive value associated with HS). During conditioning, rats receive HS in one chamber and NS in the other chamber. After conditioning, rats spend less time in the chamber paired with HS during the post conditioning phase (blue bars) than during the preconditioning phase (white bars), and more time in the chamber paired with NS. n = 14; P < 0.0001; paired Student’s t-test. - At the end of the test, place the animal back in his cage; clean the apparatus, and mesh table with a 70% ethanol solution.
- For optogenetic manipulation of neuronal activity, prior to conducting the behavioral experiments, perform transcranial injections of optogenetic constructs. Anesthetize animals with isoflurane (1.5 to 2%). In all experiments, the virus is delivered to the anterior cingulate cortex (ACC) only. Rats are bilaterally injected with 0.5 μl of viral vectors at a rate of 0.1 μl/10 sec with a 26-G 5 μl Hamilton syringe at anteroposterior (AP) +2.6 mm, mediolateral (ML) ±1.6 mm, and dorsoventral (DV) -2.25 mm, with tips angled 28° toward the midline. The microinjection needles are left in place for 10 min, raised 1 mm and left for another minute to allow for diffusion of virus particles away from injection site, while minimizing spread of viral particles along the injection tract.
Data analysis
The data analysis was conducted using GraphPad Prism version 7. The ANY-maze software provides us a value of time spent in each chamber and we analyze the videos offline to be sure that the tracking was efficient, and not erroneous because of tracking artifacts introduced by, for example, manual movement of the laser fiber.
A paired Student’s t-test was used to compare the time spent in each treatment chamber before and after conditioning (i.e., baseline vs. test phase for each chamber). Decreased time spent in a chamber during the test phase when compared with the baseline, indicates avoidance (aversion) for that chamber.
Notes
- All procedures in this study were approved by the New York University School of Medicine Institutional Animal Care and Use Committee (IACUC) as consistent with the National Institute of Health Guide for the care and use of laboratory Animals to ensure minimal animal use and discomfort. Male Sprague-Dawley rats were purchased from Taconic Farms, Albany, NY and kept at Mispro Biotech Services Facility in the Alexandria Center for Life Science, with controlled humidity, temperature, and 12 h (6:30 AM to 6:30 PM) light-dark cycle. Food and water were available ad libitum. Animals arrived to the animal facility at 250 to 300 g and were given on average 10 days to adjust to the new environment prior to the onset of experiments.
- The apparatus was made of 6 black acrylic plexiglas panels (2 panels 16 x 13 and 2 panels 7 x 13 to build the rectangular base, 2 panels 1 x 13 to do the separation between the 2 chambers). The panels were glued together to make a two chamber apparatus, dimensions 16 x 7 x 13 cm (see Equipment).
- The box was divided into two chambers. To allow the animal to discriminate between the 2 chambers, each one is paired with different cues (any kind of smell, visual, or tactile cues). In our experiment, we applied different smell cues inside each lateral side of the 2 chambers (right chamber = cherry fruity smell/left chamber = mint smell).
- The habituation of the rats to the experimenter 1 week prior to the test is done to ensure that the rats are comfortable with the experimenter and the testing environment. This will reduce/mitigate the effect that the presence of the experimenter and novel environment will have on the rat’s movement during the experiments. The animals were handled by the experimenter daily for 5 min, until the animals showed signs of stress (start to urinate, defecate or vocalize), or escaped from the experimenter’s arms. No force or restraint was used and the time the animals are handled increases daily. The habituation was performed in the same room as the experiments will be conducted. In addition to the experimenter’s handling, the animals were also placed 5 min in the two-chamber apparatus for habituation to the environment. The experimenter is expected to stand in a neutral position when performing the test; a location where each chamber is equidistant from the experimenter. All the phases of the experiment (preconditioning, conditioning, and testing) should occur, one right after the other.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM115384), National Institute of Neurological Disorders and Stroke (NS100065), (Bethesda, MD, USA) and the Anesthesia Research Fund of New York University Department of Anesthesiology (New York, NY, USA). The authors declare no competing financial interests. This protocol was adapted from LaBuda and Fuchs, 2000.
References
- Ding, H. K., Shum, F. W., Ko, S. W. and Zhuo, M. (2005). A new assay of thermal-based avoidance test in freely moving mice. J Pain 6(7): 411-416.
- He, Y., Tian, X., Hu, X., Porreca, F. and Wang, Z. J. (2012). Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain 13(6): 598-607.
- Johansen, J. P., Fields, H. L. and Manning, B. H. (2001). The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 98(14): 8077-8082.
- LaBuda, C. J. and Fuchs, P. N. (2000). A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163(2): 490-494.
- LaGraize, S. C., Labuda, C. J., Rutledge, M. A., Jackson, R. L. and Fuchs, P. N. (2004). Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol 188(1): 139-148.
- McNabb, C. T., Uhelski, M. L. and Fuchs, P. N. (2012). A direct comparison of affective pain processing underlying two traditional pain modalities in rodents. Neurosci Lett 507(1): 57-61.
- Qu, C., King, T., Okun, A., Lai, J., Fields, H. L. and Porreca, F. (2011). Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 152(7): 1641-1648.
- van der Kam, E. L., De Vry, J., Schiene, K. and Tzschentke, T. M. (2008). Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain 136: 373-79.
- Zhang, Q., Manders, T., Tong, A. P., Yang, R., Garg, A., Martinez, E., Zhou, H., Dale, J., Goyal, A., Urien, L., Yang, G., Chen, Z. and Wang, J. (2017). Chronic pain induces generalized enhancement of aversion. Elife 6: e25302.
Article Information
Copyright
Urien et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Urien, L., Zhang, Q., Martinez, E., Zhou, H., Desrosier, N., Dale, J. and Wang, J. (2017). Assessment of Aversion of Acute Pain Stimulus through Conditioned Place Aversion. Bio-protocol 7(21): e2595. DOI: 10.21769/BioProtoc.2595.
- Zhang, Q., Manders, T., Tong, A. P., Yang, R., Garg, A., Martinez, E., Zhou, H., Dale, J., Goyal, A., Urien, L., Yang, G., Chen, Z. and Wang, J. (2017). Chronic pain induces generalized enhancement of aversion. Elife 6.
Category
Neuroscience > Behavioral neuroscience > Animal model > Rat
Neuroscience > Sensory and motor systems > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









