- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Touchscreen-based Visual Discrimination and Reversal Tasks for Mice to Test Cognitive Flexibility
Published: Vol 7, Iss 20, Oct 20, 2017 DOI: 10.21769/BioProtoc.2583 Views: 10562
Reviewed by: Soyun KimAlexandra GrosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4459 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4569 Views

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1772 Views
Abstract
Reversal learning can be used to examine deficits in cognitive flexibility, which have been linked to a number of neuropsychiatric disorders including schizophrenia and addiction. However, methods of examining reversal learning have varied substantially between species. Touchscreen technology has allowed researchers to explore cognitive deficits with a platform that is translatable across rodents, non-human primates and human subjects. Here we describe a method for measuring visual discrimination and reversal learning in mice using automated touchscreen-based operant chambers.
Keywords: Visual discriminationBackground
Cognitive flexibility is the ability to flexibly adjust responses to a previously learned stimulus-reward association, and impairments occur in a range of neuropsychiatric conditions, including schizophrenia, autism, obsessive-compulsive disorder and addiction. To further study the neural mechanisms implicated in cognitive flexibility, performance of choice and reversal tasks have been used in animal models. There are a variety of methods used to measure cognitive flexibility in rodent models, however many techniques have been difficult to compare between rodent and human studies (Brigman et al., 2010). However, using touchscreens, a similar paradigm can be used to study reversal learning across species (Bussey et al., 2012; Horner et al., 2013). Trained rodents are able to discriminate visual stimuli and then successfully reverse their choice when the contingency changes. However, cognitive performance in both rats and mice has been shown to be highly strain dependent (Graybeal et al., 2014). C57BL/6J mice are a popular strain for behavioural and genetic studies, and have been used as a standard strain against which others are compared (Izquierdo et al., 2006). Meanwhile, BALB/c mice often display poor learning and cognitive performance compared to other strains (Graybeal et al., 2014). Recently, the BALB/c strain was shown to be ‘severely impaired’ in basic training, visual discrimination and reversal learning using touchscreen chambers (Graybeal et al., 2014). Therefore, we have adapted the training protocol to promote responding in an anxious mouse strain (BALB/c), where behavioural (rather than cognitive) traits may impair performance. Our results indicated that this protocol provides comparable levels of performance in the standard C57BL/6 mouse to those previously published and significantly enhanced performance of the anxious and emotionally reactive BALB/c mouse (Turner et al., 2017).
Materials and Reagents
- Small containers, such as 50 ml Falcon tube lids (BD Biosciences)
- Paper towels for cleaning chambers
- Our experiment used male BALB/c or C57BL/6J mice (Animal Resource Centre, Australia) at 12 weeks of age one week after arrival to allow the mice to habituate to the facility
Notes:- Mice are housed in groups of four in individually ventilated cages (OptiMICE, Animal Care Systems, USA) with water available ad libitum. Mice are housed with bedding with tissues. The temperature (21 ± 1 °C) and humidity (50 ± 10%) are controlled and lights are kept on a 12-h cycle (lights on at 07:00 AM).
- Mice are tail marked for identification and weighed for 3 days to get an average free-feeding body weight. Food restriction should be conducted for at least 3 days prior to testing to gradually reduce weight and allow mice to adapt to a set feeding schedule. Food restriction is used as mice will readily perform for strawberry milk rewards when hungry.
- They are then food restricted to around 90% of their free-feeding body weight using small pieces of food to minimise fighting.
Ensure that growth relevant to the strain and age is considered in determining ongoing food restriction limits. - Treatment group size should be based on a power analysis where possible, however groups of 10-15 mice are commonly considered sufficient.
- Mice are housed in groups of four in individually ventilated cages (OptiMICE, Animal Care Systems, USA) with water available ad libitum. Mice are housed with bedding with tissues. The temperature (21 ± 1 °C) and humidity (50 ± 10%) are controlled and lights are kept on a 12-h cycle (lights on at 07:00 AM).
- Undiluted strawberry milk (Breaka, Parmalat, Australia)
- Ethanol (70%) for cleaning chambers
Equipment
- Bussey-Saksida Mouse Touchscreen Chambers (Campden Instruments, model: Model 80614 ) equipped with:
- Touchscreen
- House light
- Liquid reward dispenser
- Magazine
- Two-window black masks
- Overhead camera
- Sound-attenuating chamber
- Multi-Media Single Station Licence for each chamber (Campden Instruments, model: Model 80698-1 )
- Touchscreen
Software
- PC with ABET II Software for Touchscreens (Model 89505, Campden Instruments Ltd., UK) with Whisker Multi-Media Single Station Licence for each chamber (Model 80698-1, Campden Instruments Ltd., UK)
- SPSS (ver.20, SPSS Inc., Chicago, USA)
Procedure
Animal Ethics Approval: Researchers must determine ethical approvals required and maintain compliance with relevant university and government guidelines regarding the care and use of laboratory animals prior to starting experiments.
- Operant training
- Prior to training, mice are exposed to the strawberry milk reward by providing small amounts (~0.5 ml) in the home cage. A small autoclavable container (e.g., 50 ml Falcon tube lid) was half filled with milk and placed in the home cage for three consecutive days before training commenced. Mice may bury the lids rather than consume the milk initially, however three days seems sufficient to introduce the reward and reduce neophobia.
- Prior to transporting the mice the operant chamber equipment should be checked and prepared for testing. The mask should be placed over the touchscreens and the reward lines should be primed with fresh strawberry milk.
- Mice are then transported to the dimly lit (< 10 lux) testing room in their home cages and allowed 30 min to habituate each day before testing.
- The data and program are loaded on the computer, and then each mouse is placed into the correct operant chamber for testing. Mice are always tested in the same operant chamber because contextual changes can impair learning. Testing should occur at the same time each day and for at least 5 days per week. Feeding should also occur at the same time each day, but at least 1 h after testing has finished.
- Prior to training, mice are exposed to the strawberry milk reward by providing small amounts (~0.5 ml) in the home cage. A small autoclavable container (e.g., 50 ml Falcon tube lid) was half filled with milk and placed in the home cage for three consecutive days before training commenced. Mice may bury the lids rather than consume the milk initially, however three days seems sufficient to introduce the reward and reduce neophobia.
- Training stages
Several steps (5 pre-training and 2 task steps) are used during training. Mice can pass to the next step if the criteria were fulfilled. The training parameters and criteria to move to the next stage are shown in Table 1. The image that is rewarded should be counterbalanced within each treatment group, but constant for an individual mouse. Within each group, half of the mice are required to respond to one or the other stimulus to receive rewards. We have used one session per day, but multiple sessions can be run in a day, depending on the number of animals in a cohort or on the number of chambers that are available. Therefore, criteria are based on the number of sessions completed.
Table 1. Parameters and criteria for the training stages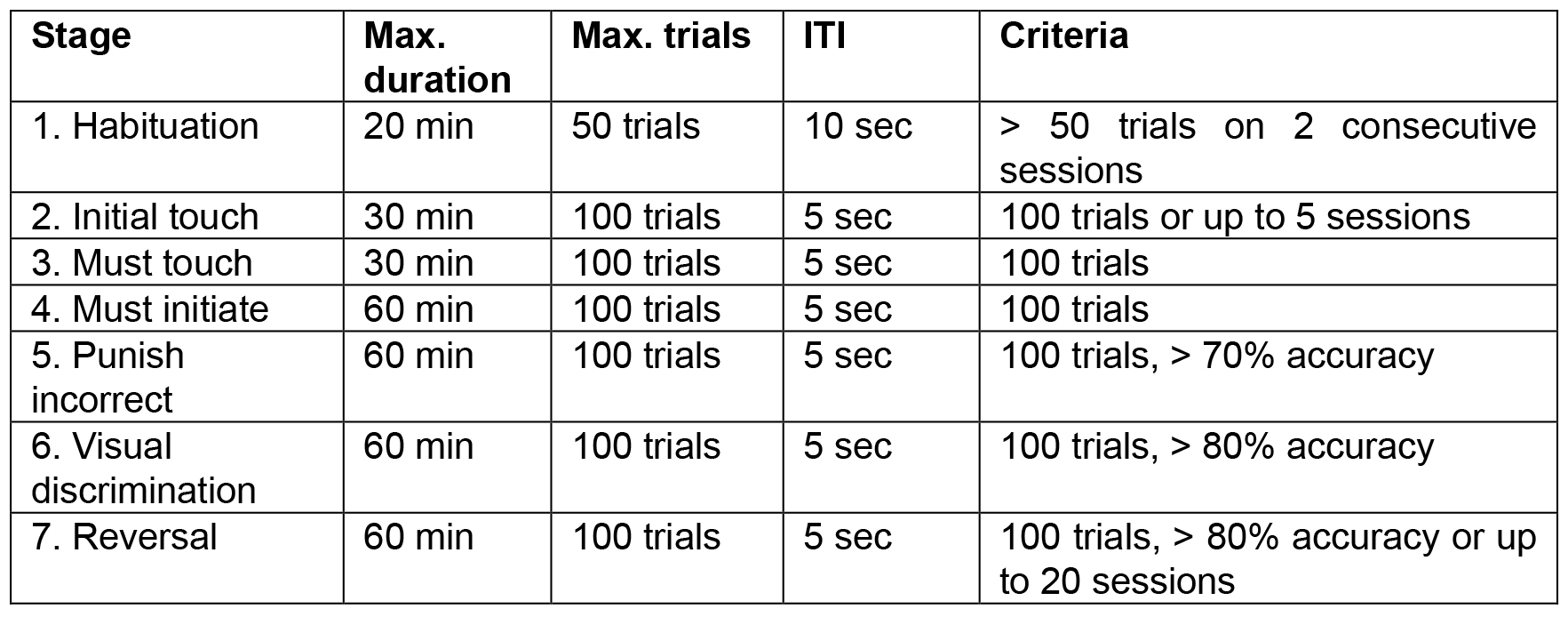
- Pre-training: Habituation
The mice learn to collect rewards from the illuminated magazine. The reward is pumped for 6,000 msec (approx. 150 μl) and the magazine light is illuminated. Once the head entry is detected, the light turns off and after the head is removed the inter-trial interval (ITI) begins (10 sec). After the ITI, a single reward (280 msec) is delivered and the magazine light illuminated, start the cycle again (Figure 1.1). - Pre-training: Initial Touch
The mouse is rewarded for touching the white square on the touchscreen. Two images are selected pseudorandomly to appear in each location. One image is a white square and the other is a blank, black screen. If the square is touched within 30 sec of display, it is removed and 3 x reward (840 msec) is delivered to an illuminated magazine. If there are no touches for 30 sec, then a single reward is delivered instead (280 msec). In both cases, removal of the head from the magazine starts the ITI (5 sec) before the next trial starts. Touches to the blank image are recorded but have no consequences (Figure 1.2). - Pre-training: Must Touch
The mice must touch the white square on the touchscreen to receive a reward. As for Must Touch, a white square will appear in one location and the mouse must touch it to receive a single reward and no reward is given for omitting a response. Blank touches have no consequences (Figure 1.3). - Pre-training: Must Initiate
The mice must start the trials by nose poking in the magazine. The protocol starts as per Must Touch with the presentation of the same images where touching the white square is rewarded and blank touches have no consequences. After reward collection, the magazine light is illuminated again and a head entry is required to proceed to the next trial (Figure 1.4). - Pre-training: Punish Incorrect
The mice receive a time-out for touching the incorrect location. As per Must Initiate, however now touching the blank image results in a time-out (5 sec) when the house light is illuminated (Figure 1.5). - Task: Visual Discrimination (VD)
Mice must discriminate between two visual stimuli and respond to the correct image. The protocol is very similar to Punish Incorrect, except that the testing stimuli pair is now used (Figure 1.6). The stimuli used have black backgrounds with either a single large white circle or four small white circles in each corner of the image (Figure 2).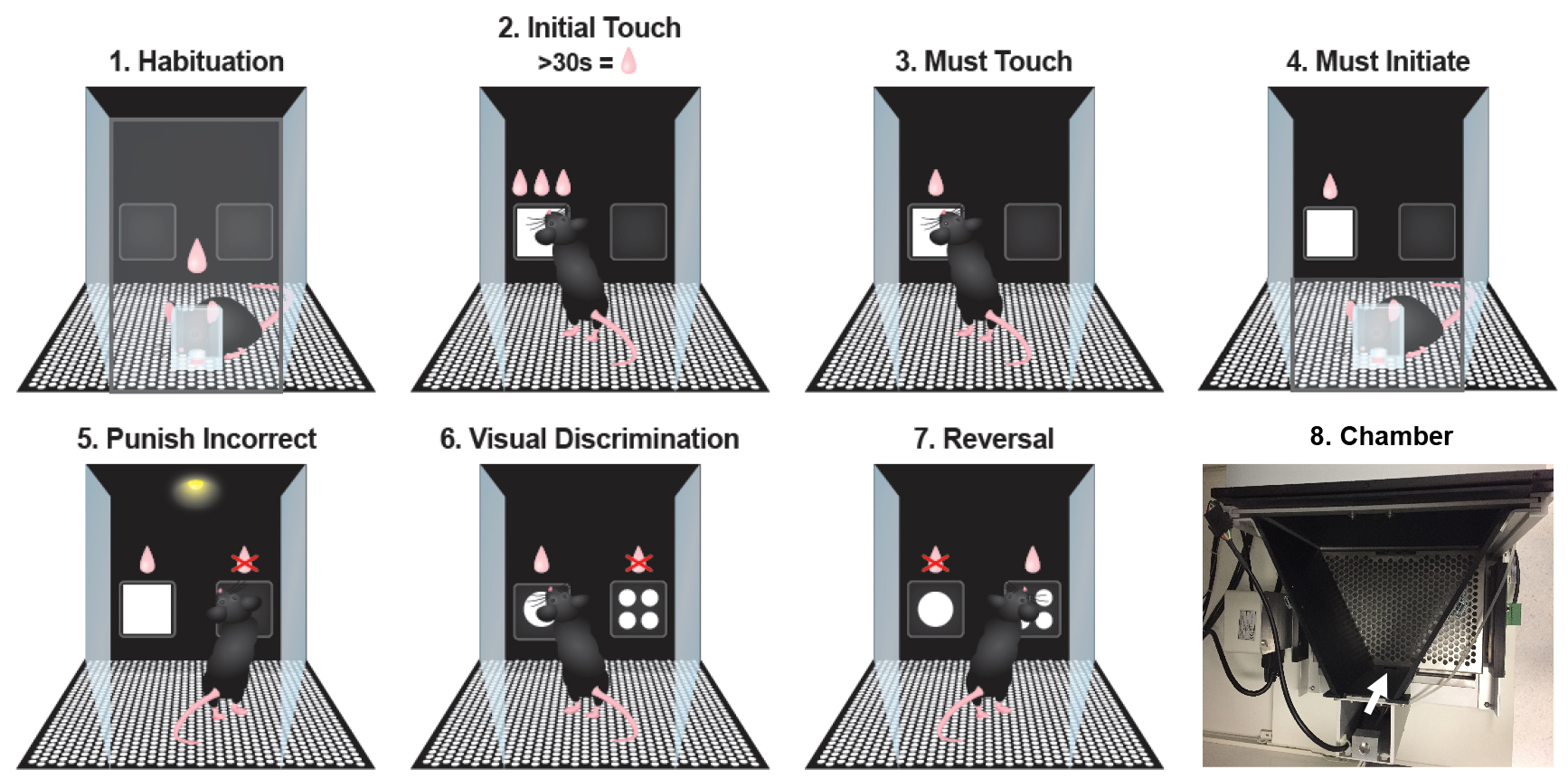
Figure 1. Visual discrimination and reversal training stages. 1. Habituation where the mouse learns to collect rewards; 2. Initial touch where the mouse is rewarded with 3 rewards for touching the white square on the screen or 1 reward after 30 sec. 3. Must touch where the mouse will only receive a reward for touching the white square on the screen. 4. Must initiate where the mouse must start trials with a magazine nose poke. 5. Punish incorrect where the mouse now receives a time-out for incorrectly touching the blank screen. 6. Visual discrimination where the mouse learns to select the correct image (large circle in example) to receive a reward. 7. Reversal where the mouse must now select the alternative image (small circles in example) to receive a reward. 8. Photograph of top view of operant chamber to show stimulus panel towards top of the image and reward collection port (indicated by white arrow).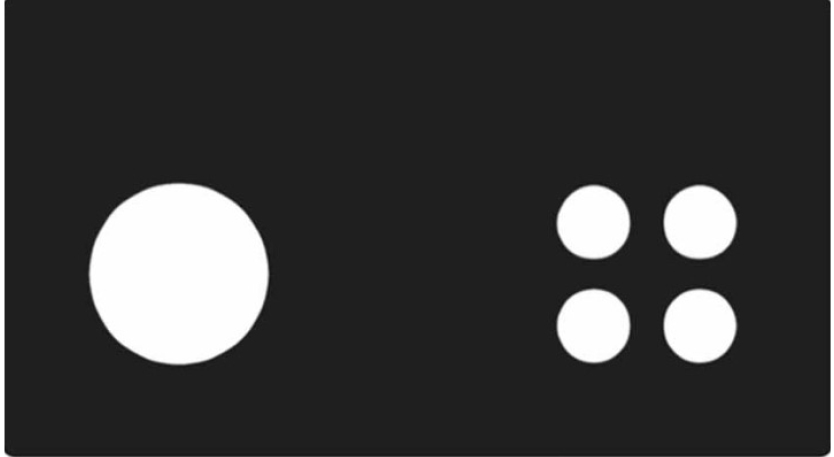
Figure 2. Visual discrimination stimuli. The images used in this protocol were a single large white circle versus four small white circles. These stimuli were selected as they have similar graphic features and would allow the use of orientation shifted patterns (such as vertical vs. horizontal lines) within a battery of other tasks. - Task: Reversal
The stimuli that were previously paired with reward should now be avoided and the alternative stimuli (previously unrewarded stimuli) are now correct (Figure 1.7).
- Pre-training: Habituation
Data analysis
Statistical analysis is conducted using SPSS (ver.20, SPSS Inc., Chicago, USA) with significance set at P < 0.05. The primary measures include trials completed, response accuracy (correct, incorrect or blank touch), the number of correction trials, session duration, response and reward latencies, response omission and the number of sessions required on each training stage to reach criteria. Correction trials repeat the previous stimulus in the same location until the correct stimulus/location is selected. This is very important when first training VD to prevent the mouse from simply selecting one location and being rewarded on 50% of trials. Other measures include irrelevant touches to the screen during the inter-trial interval (ITI) or time-out periods, and the number of fronts and rear beam breaks as a measure of activity. The effects of mouse strain, drug treatment or other manipulation on these measures can be examined by independent or paired t-tests, ANOVA or repeated measures ANOVA where appropriate to the experimental design and with consideration for multiple comparisons.
Notes
Representative results from two mouse strains (examples in Videos 1 and 2) can be seen in Table 2. The average number of sessions to reach criteria are given for each of the stages (5 pre-training and 2 task steps). Under these testing conditions, BALB/c mice take fewer sessions to complete the task. However, the percent correct responses for VD and reversal and the reward latency did not differ between the mouse two strains.
Table 2. Example of results from two commonly used mouse strains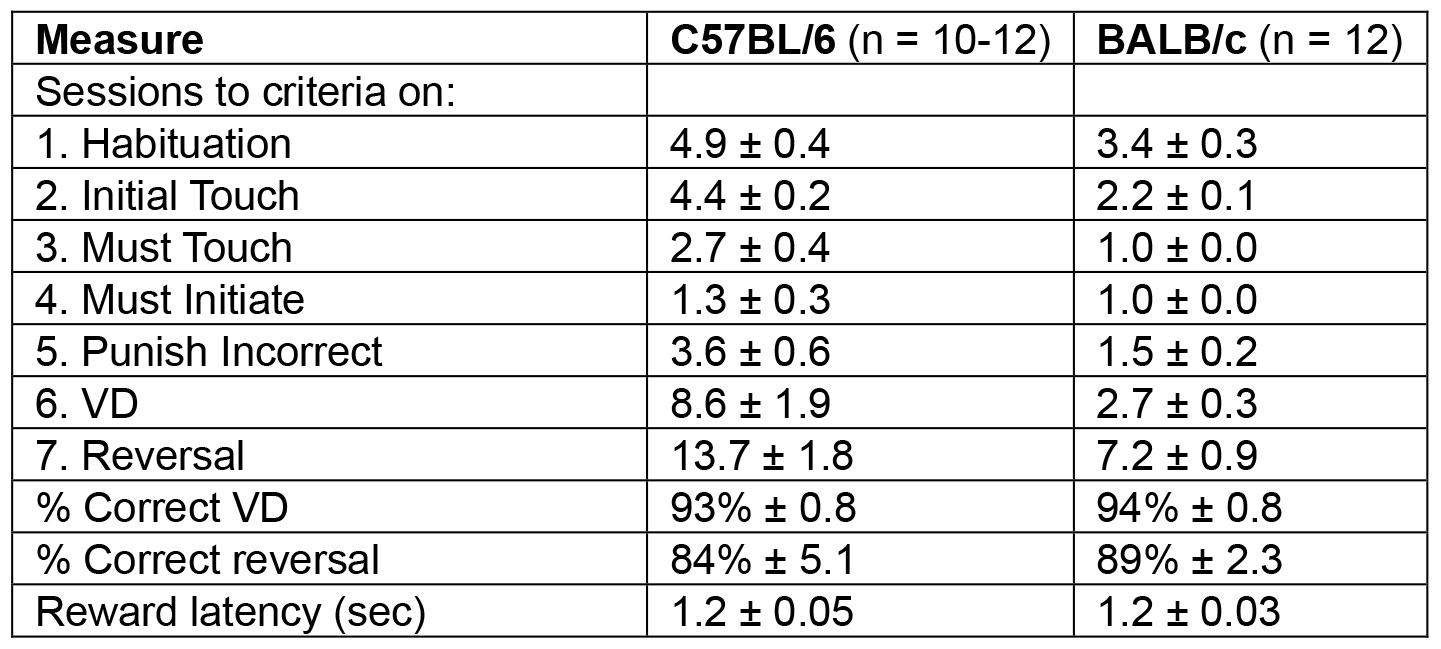
Acknowledgments
This procedure was adapted from published protocols (Izquierdo et al., 2006; Horner et al., 2013; Graybeal et al., 2014) and full results of the mouse strain comparison using this protocol can be found in Turner et al. (2017). This work was funded by the National Health and Medical Research Council of Australia and a University of Queensland Major Equipment and Infrastructure Grant to TB. KT was supported by the Queensland Government Smart Futures PhD Scholarship and an Australian Postgraduate Award. Figures were produced by Nick Valmas.
References
- Brigman, J. L., Graybeal, C. and Holmes, A. (2010). Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci 4.
- Bussey, T. J., Holmes, A., Lyon, L., Mar, A. C., McAllister, K. A., Nithianantharajah, J., Oomen, C. A. and Saksida, L. M. (2012). New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62(3): 1191-1203.
- Graybeal, C., Bachu, M., Mozhui, K., Saksida, L. M., Bussey, T. J., Sagalyn, E., Williams, R. W. and Holmes, A. (2014). Strains and stressors: an analysis of touchscreen learning in genetically diverse mouse strains. PLoS One 9(2): e87745.
- Horner, A. E., Heath, C. J., Hvoslef-Eide, M., Kent, B. A., Kim, C. H., Nilsson, S. R., Alsio, J., Oomen, C. A., Holmes, A., Saksida, L. M. and Bussey, T. J. (2013). The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc 8(10): 1961-1984.
- Izquierdo, A., Wiedholz, L. M., Millstein, R. A., Yang, R. J., Bussey, T. J., Saksida, L. M. and Holmes, A. (2006). Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res 171(2): 181-188.
- Turner, K. M., Simpson, C. G. and Burne, T. H. (2017). BALB/c mice can learn touchscreen visual discrimination and reversal tasks faster than C57BL/6 mice. Front Behav Neurosci 11: 16.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Turner, K. M., Simpson, C. and Burne, T. H. J. (2017). Touchscreen-based Visual Discrimination and Reversal Tasks for Mice to Test Cognitive Flexibility. Bio-protocol 7(20): e2583. DOI: 10.21769/BioProtoc.2583.
Category
Neuroscience > Behavioral neuroscience > Cognition
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









