- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Scanning Electron Microscopy of Motile Male Gametes of Land Plants
Published: Vol 7, Iss 19, Oct 5, 2017 DOI: 10.21769/BioProtoc.2570 Views: 8400
Reviewed by: Scott A M McAdamAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2323 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1691 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 758 Views
Abstract
The only motile cells produced in land plants are male gametes (spermatozoids), which are reduced to non-flagellated cells in flowering plants and most gymnosperms. Although a coiled architecture is universal, the complexity of land plant flagellated cells varies from biflagellated in bryophytes to thousands of flagella per gametes in the seed plants Ginkgo and cycads. This wide diversity in number of flagella is associated with vast differences in cell size and shape. Scanning electron microscopy (SEM) has played an important role in characterizing the external form, including cell shape and arrangement of flagella, across the varied motile gametes of land plants. Because of the size and scarcity of released swimming sperm, it is difficult to concentrate them and prepare them for observation in the SEM. Here we detail an SEM preparation technique that yields good preservation of sperms cells across plant groups.
Keywords: FlagellaBackground
Motile gametes of land plants are strikingly diverse and develop through transformations that involve repositioning cellular components and the assembly of a complex locomotory apparatus (Renzaglia and Garbary, 2001). Because of constraints imposed by cell walls, elongation of the cell and flagella is around the periphery of a nearly spherical space, resulting in a coiled configuration of the mature gamete. The degree of coiling varies from just over one to as many as 10 revolutions per cell. The number of flagella per gamete is even more variable, ranging from two in bryophytes (mosses, hornworts, and liverworts) to an estimated 1,000-40,000 in Ginkgo and cycads. Following the diversification of Ginkgo and cycads, all vestiges of basal bodies and flagella were lost in the remaining seed plants that utilize pollen tubes to deliver non-motile sperm to egg cells (Southworth and Cresti, 1997). Male gametes provide a wealth of biological information, including biodiversity and cell differentiation and evolution (Garbary et al., 1993; Renzaglia et al., 1995; Renzaglia and Garbary, 2001; Renzaglia et al., 2000; Lopez-Smith and Renzaglia, 2008; Lopez and Renzaglia, 2014). Of the range of microscopic techniques utilized to characterize plant spermatozoids, SEM provides the most direct means of elucidating cell shape, and flagella number, length and arrangement. Together with TEM observations, SEM studies lead to comparative descriptions of gamete architecture, and organellar content and arrangement across plant lineages (Renzaglia et al., 2001 and 2002; Lopez-Smith and Renzaglia, 2008).
Materials and Reagents
Scanning Electron Microscopy (SEM) and the motile sperm cell architecture
- Pipette tips 1-200 µl (Carolina Biological Supply, catalog number: 215055 )
- Pasteur pipettes (Fisher Scientific, catalog number: 13-678-4)
Manufacturer: Corning, catalog number: C7095B5X .
- 1.5-ml centrifuge tubes (USA Scientific, catalog number: 1615-5500 )
- Glass coverslips 22 x 22 mm (Fisher Scientific, catalog number: 12-542B )
- Male plants with mature sperm cells
- Pellia epiphylla
- Conocephalum conicum
- Equisetum arvense
- Ceratopteris richardii
- Pellia epiphylla
- Sorensens phosphate buffer, 0.2 M, pH 7.2 (Electron Microscopy Sciences, catalog number: 11600-10 )
- Glutaraldehyde (Electron Microscopy Sciences, catalog number: 16120 )
- Sodium cacodylate buffer, 0.2 M, pH 7.4 (Electron Microscopy Sciences, catalog number: 11652 )
- Osmium tetroxide (Electron Microscopy Sciences, catalog number: 19150 )
- Ethanol (Decan Laboritories, catalog number: 2705HC )
- Hexamethyl disilizane (HMDS) (Electron Microscopy Sciences, catalog number: 16700 )
- 0.01 M phosphate buffer (pH 7.2) (see Recipes)
- 0.05 M phosphate buffer (pH 7.2) (see Recipes)
- 0.02 M phosphate buffer (pH 7.2) (see Recipes)
- 2.5% glutaraldehyde (see Recipes)
- 0.05 M sodium cacodylate buffer (pH 7.2) (see Recipes)
- 2% aqueous osmium tetroxide (see Recipes)
Equipment
- Scanning electron microscope (SEM) (FEI, model: QuantaTM 450 FEG )
- Shaker (Thermolyne, model: M-16715 )
- Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: mySPINTM 6 , catalog number: 75004061)
- Oven (General Signal, model: Gravity convection )
- SEM Specimen Mount Stubs, Aluminum Slotted Head (Electron Microscopy Sciences, catalog number: 75220 )
- Samdri 790 Critical Point Dryer (Tousumis)
- Sputter coater (Denton Vacuum, model: Desk V )
Procedure
Scanning Electron Microscopy (SEM) and the motile sperm cell architecture:
SEM examination of mature sperm cells has enabled rapid visualization and comparisons of whole gamete architecture compared with laborious TEM observations and cell reconstructions. The diversity of gamete architecture and organization of flagella in mature cells is readily seen in the SEM (Figure 1).
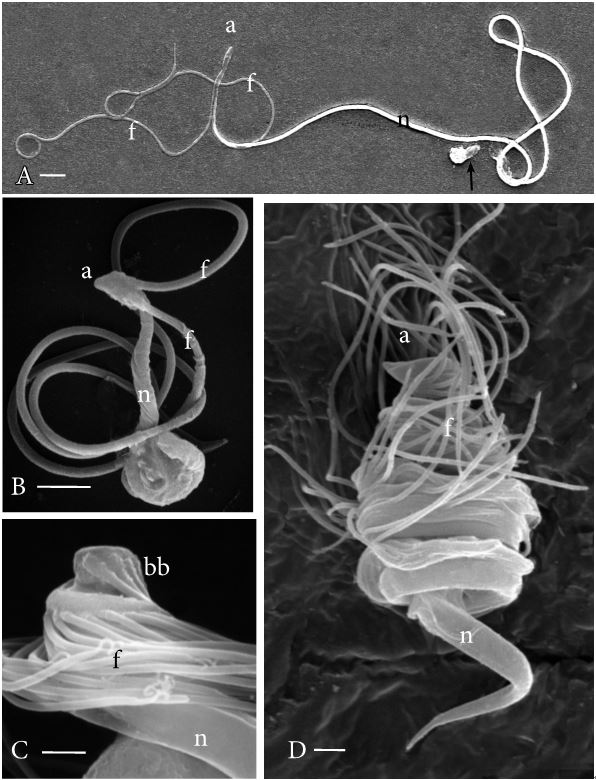
Figure 1. Scanning electron micrographs of plant sperm cells. A. Pellia epiphylla, a simple thalloid liverwort. This is one of the longest sperm cells produced by bryophytes. The two flagella (f) are staggered in their insertion near the cell anterior (a) and the cell body consists of a long cylindrical nucleus (n) with a plastid and mitochondrion at the posterior (arrow). B. Conocephalum conicum, a complex thalloid liverwort. This sperm cell is much smaller than that of Pellia. The two flagella (f) are inserted anteriorly (a) and are much longer than the cell body, which consists of a cylindrical nucleus (n) and posterior cytoplasm that in this case is not fully extended. C. Equisetum arvense, a eusporangiate fern. The approximately 36 basal bodies (bb) attach flagella (f) along the anterior coils of the cell. The nucleus (n) extends to the cell posterior (not visible). D. Ceratopteris richardii, a leptosporangiate fern. This cell coils four times and has about 80 flagella (f) inserted anteriorly (a). The cylindrical nucleus (n) extends over three coils and tapers at the cell posterior. Bars = 5.0 µm (A), 1.0 µm (B, C, D).
- Using excised male tissue (antheridia of male gametophytes), place several antheridia in each droplet (ca. 100 µl) of 0.01 M phosphate buffer, pH 7.2 (see Recipes), on a dental wax plate, cover to retard evaporation, and leave overnight at room temperature to facilitate release of sperm. Alternatively, excised antheridia can be placed in 1.5 ml Eppendorf centrifuge tubes adding 1 ml of 0.01 M phosphate buffer, pH 7.2 to completely cover tissue and left overnight. Antheridial tissue can be removed and discarded the next day. Swimming sperm will be in the buffer.
- Remove tissue and place the droplet in a 1.5 ml Eppendorf centrifuge tube containing 1 ml 2.5% glutaraldehyde (see Recipes) in 0.05 M sodium cacodylate buffer, pH 7.2 (see Recipes).
- Shake tube gently and fix the spermatozoid suspension for 1 h at room temperature.
Note: From this point on, spermatozoids are concentrated by centrifugation at 100 x g for 5 min between each solution change. Because the sperm are colorless, you will most likely not see a pellet after centrifugation.
- Remove the glutaraldehyde supernatant down to 0.1 ml leaving the pellet at bottom of tube. Rinse fixed sperm cells three times (10 min each) in 1 ml 0.05 M sodium cacodylate buffer.
- Post-fix tissue for 20 min in 1 ml 2% aqueous osmium tetroxide (see Recipes).
- Rinse specimens twice in 1 ml double distilled autoclaved water (10 min), once each in 25%, 50% and in 75% ethanol (10 min), twice in 100% ethanol (10 min), and then twice in 100% hexamethyl disilizane (HMDS; 10 min).
- Pipet sperm cells in 1 ml HMDS on to a glass coverslip affixed to a standard aluminum pin-type mounting stub and dry for 20 min in a 60 °C oven.
- Specimens on mounting stubs are sputter-coated with an ultra-thin layer (ca. 390 nm) of a conducting metal (palladium–gold) that prevents charging from non-conducting biological specimens, increases the number of secondary electrons from the specimen surface that improves topographical contrast, and minimizes damage to the specimen.
Basic steps:- Vacuum down sputter coater
- Out-gas with Argon gas
- Apply voltage
- Open gas to the specific emissions
- Coat with ca. 390 nm palladium–gold
- Vacuum down sputter coater
- View on a high-resolution SEM.
Note: Often cells aggregate around the edges of the coverslip, so it is better to put a very small drop of sperm cell suspension in HMDS in the middle of the coverslip to dry.
Recipes
- 0.01 M phosphate buffer (pH 7.2)
Add 5 parts 0.20 M Sorenson’s phosphate buffer (pH 7.2) to 95 parts double distilled autoclaved water
- 0.05 M phosphate buffer (pH 7.2)
Add 25 parts 0.20 M Sorenson’s phosphate buffer (pH 7.2) to 75 parts double distilled autoclaved water
- 0.02 M phosphate buffer (pH 7.2)
Add 1 part 0.20 M Sorenson’s phosphate buffer (pH 7.2) to 9 parts double distilled autoclaved water
- 2.5% glutaraldehyde
- Add 1 part 10% glutaraldehyde to 1 part double distilled autoclaved water
- Add 1 part 0.20 M Sorensen’s phosphate buffer (pH 7.2) to 1 part double distilled autoclaved water
- Add 1 part 5% glutaraldehyde to 1 part 0.10 M Sorensen’s phosphate buffer (pH 7.2)
- Add 1 part 10% glutaraldehyde to 1 part double distilled autoclaved water
- 0.05 M sodium cacodylate buffer (pH 7.2)
Add 1 part 0.30 M sodium cacodylate buffer (pH 7.2) to 5 parts double distilled autoclaved water
- 2% aqueous osmium tetroxide
Add 1 part 4% aqueous osmium tetroxide to 1 part double distilled autoclaved water
Acknowledgments
This research was supported by research grants (DEB-0322664, DEB-0423625, DEB0521177, and DEB-0228679) from the National Science Foundation as part of the Research Experience for Undergraduates and Assembling the Tree of Life Programs.
References
- Garbary, D. J., Renzaglia, K. S. and Duckett, J. G. (1993). The phylogeny of land plants: a cladistic analysis based on male gametogenesis. Plant Syst Evol 188: 237-269.
- Lopez, R. A. and Renzaglia, K. S. (2014). Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development. Am J Bot 101(12): 2052-2061.
- Lopez-Smith, R. and Renzaglia, K. (2008). Sperm cell architecture, insemination, and fertilization in the model fern, Ceratopteris richardii. Sex Plant Reprod 21:153-167.
- Renzaglia, K. S., Dengate, S. B., Schmitt, S. J., Duckett, J. G. (2002). Novel features of Equisetum arvense spermatozoids: insights into pteridophyte evolution. New Phytol 154:159-174.
- Renzaglia, K. S., Duff, R. J. T., Nickrent, D. L. and Garbary, D. J. (2000). Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philos Trans R Soc Lond B Biol Sci 355(1398): 769-793.
- Renzaglia, K. S. and Garbary, D. J. (2001). Motile male gametes of land plants: Diversity, development, and evolution. Crit Rev Sci 20:107-213.
- Renzaglia, K. S., Johnson, T. H., Gates, H. D. and Whittier, D. P. (2001). Architecture of the sperm cell of Psilotum. Am J Bot 88(7): 1151-1163.
- Renzaglia, K. S., Rasch, E. M. and Pike, L. M. (1995). Estimates of nuclear DNA content in bryophyte sperm cells: phylogentic considerations. Am J Bot 82: 18-25.
- Southworth, D. and Cresti, M. (1997). Comparison of flagellated and nonflagellated sperm in plants. Am J Bot 84:1301-1311.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Renzaglia, K. S., Lopez, R. A. and Schmitt, S. J. (2017). Scanning Electron Microscopy of Motile Male Gametes of Land Plants. Bio-protocol 7(19): e2570. DOI: 10.21769/BioProtoc.2570.
- Renzaglia, K. S., Villarreal, J. C., Piatkowski, B. T., Lucas, J. R. and Merced, A. (2017). Hornwort Stomata: Architecture and Fate Shared with 400-Million-Year-Old Fossil Plants without Leaves. Plant Physiol 174(2): 788-797.
Category
Cell Biology > Cell imaging > Electron microscopy
Plant Science > Plant cell biology > Cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link