- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Xyloglucan Composition in Arabidopsis Leaves
Published: Vol 7, Iss 19, Oct 5, 2017 DOI: 10.21769/BioProtoc.2569 Views: 7101
Reviewed by: Marisa RosaLifeng LiuHarrie van Erp

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1896 Views

New Approach to Detect and Isolate Rhamnogalacturonan-II in Arabidopsis thaliana Seed Mucilage
Dayan Sanhueza and Susana Saez-Aguayo
Sep 5, 2025 1248 Views

Detailed Method for the Purification of Rhamnogalacturonan-I (RG-I) in Arabidopsis thaliana
Liang Zhang [...] Breeanna R. Urbanowicz
Feb 5, 2026 144 Views
Abstract
Xyloglucan is one of the main components of the primary cell wall in most species of plants. This protocol describes a method to analyze the composition of the enzyme-accessible and enzyme-inaccessible fractions of xyloglucan in the model species Arabidopsis thaliana. It is based on digestion with an endoglucanase that attacks unsubstituted glucose residues in the backbone. The identities and relative amounts of released xyloglucan fragments are then determined using MALDI-TOF mass spectrometry.
Keywords: XyloglucanBackground
In many flowering plants xyloglucan is a major component of primary cell walls, where it plays an important role in growth regulation. Sequential extraction protocols offer a way of separating distinct xyloglucan domains (Pauly et al., 1999). Some xyloglucan appears to be trapped inside cellulose microfibrils while another fraction is bound to their surface through hydrogen bonding. The rest of the xyloglucan, possibly the majority of it, occupies the space between microfibrils (Park and Cosgrove, 2015). Part of this later xyloglucan can be extracted through direct endoglucanase digestion of cell wall material. Much of the remaining xyloglucan can then be released through alkaline treatment. Studies of mutants deficient in xyloglucan exoglycosidases have shown that these enzymes, together with Xyloglucan Endotransglycosylases/Hydrolases, act mostly on the enzyme-accessible fraction (Sampedro et al., 2010; Günl and Pauly, 2011; Günl et al., 2011; Sampedro et al., 2012; Sampedro et al., 2017). Digestion of Arabidopsis xyloglucan with endoglucanases that attack unsubstituted glucose residues results in a mixture of three and four-glucose subunits that can be quickly and easily analyzed through MALDI-TOF mass spectrometry (Lerouxel et al., 2002; Günl et al., 2010). The area of the ion peaks can be then used to quantify the abundance of the different fragment, although there is evidence of significant differences in response factors (Tuomivaara et al., 2015). This protocol incorporates some changes from our previous versions, such as the use of SDHB (Super-DHB) matrix, which reduces the noise, and the addition of NaCl during extraction to prevent the formation of potassium adducts.
Materials and Reagents
- Pipette tips (2 μl, 200 μl, 1,000 μl)
- 1.5 ml microcentrifuge tubes
- Centrifugal filters, modified PES, 10K (VWR, catalog number: 82031-348 )
- 200 μl PCR tubes
- Mature Arabidopsis leaves (2 or 3 leaves)
- Liquid nitrogen
- Ethanol (Merck, catalog number: 1.00983 )
- Type II purified water
- Acetone (Sigma-Aldrich, catalog number: 179973 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Cellulase suspension from Trichoderma longibrachiatum (Megazyme, catalog number: E-CELTR )
- Pyridine (VWR, catalog number: 27199.292 )
- Thimerosal (Sigma-Aldrich, catalog number: T5125 )
- Sodium hydroxide (NaOH) (Merck, catalog number: 106469 )
- Acetic acid (AppliChem, catalog number: 141008.1611 )
- 2,5-Dihydroxybenzoic acid (Sigma-Aldrich, catalog number: 39319 )
- 2-Hydroxy-5-methoxybenzoic acid (Sigma-Aldrich, catalog number: 146188 )
- Acetonitrile (Merck, catalog number: 1.00029 )
- Xyloglucan oligosaccharide mixture (Megazyme, catalog number: O-XGHON )
- Digestion buffer (see Recipes)
- Super-DHB (SDHB) matrix (see Recipes)
Equipment
- Variable volume single channel manual pipettes (0.2 to 2 μl, 2 to 20 μl, 10 to 100 μl, 100 to 1,000 μl)
- Pellet pestles (Sigma-Aldrich, catalog number: Z359947 )
- Bench pillar drill, 250 W
- Insulation foam block
- Polycarbonate cover
- Dry block heater
- Vortexer
- Microcentrifuge (Eppendorf, model: 5424 )
- SpeedVac System (Thermo Fisher Scientific, Thermo ScientificTM, model: SavantTM SC210 P1 )
- Ultrasonic bath
- Orbital shaker
- MALDI target plate (Bruker, model: MTP 384 ground steel TF, catalog number: 8209519 )
- Mass spectrometer (Bruker, model: UltraFlex III MALDI-TOF/TOF )
Software
- Flex Analysis Version 3.0 (Bruker)
Procedure
- Cell wall extraction
- Collect 100 mg of mature Arabidopsis leaves (2 or 3 leaves) in a microcentrifuge tube.
Note: We usually collect fully expanded leaves with no signs of senescence from 4 to 5 weeks old plants grown in 16-h days at 22 °C/18 °C light/dark temperature.
- Grind for a few seconds with a pestle mounted on a bench pillar drill at about 600 rpm, keeping the sample submerged in liquid N2 (see Figure 1). Do not add liquid N2 to the sample. Pestles should be changed between samples.

Figure 1. Grinding setup. The bottom half of a plastic 60 ml container was inserted in a block of insulation foam and covered with a perforated piece of polycarbonate. A channel was added to facilitate addition of liquid N2.
- Add 1 ml of 80% ethanol. Vortex for 5 sec and heat for 10 min at 50 °C in a dry block heater. Centrifuge for 5 min at 3,000 x g and discard the supernatant.
- Add 1 ml of 100% ethanol to the precipitate. Vortex for 5 sec and keep at room temperature for 10 min. Centrifuge for 5 min at 3,000 x g and discard the supernatant.
- Add 1 ml of acetone to the precipitate. Vortex for 5 sec and keep at room temperature for 10 min. Centrifuge for 5 min at 3,000 x g and discard the supernatant.
- Dry samples in a Speed-Vac for 5 min approximately.
Note: We try to avoid complete drying to facilitate resuspension. Samples can also be air-dried. Dried samples can be stored at -20 °C for later processing.
- Collect 100 mg of mature Arabidopsis leaves (2 or 3 leaves) in a microcentrifuge tube.
- Extraction of enzyme-accessible xyloglucan
- Add 1 ml of 100 mM NaCl to dried wall residue and resuspend in an ultrasonic bath for at least 20 min. Vortex the sample for 5 sec several times during resuspension. Centrifuge for 3 min at 18,000 x g and discard the supernatant.
- Add 1 ml of water to cell wall residue, vortex for 5 sec and incubate in an ultrasonic bath for at least 5 min. Centrifuge for 3 min at 18,000 x g and discard the supernatant. Repeat for a total of two times.
- Add 350 μl of digestion buffer (see Recipes).
Note: Complete resuspension is not necessary as it will be finished during overnight incubation.
- Pipette a volume of cellulase suspension that contains 6 units of enzyme per sample into a centrifugal filter. Volume will vary depending on enzyme batch. Add 450 μl of digestion buffer. Centrifuge for 5 min at 14,000 x g and discard the filtrate.
- Add 450 μl of digestion buffer. Centrifuge for 5 min at 14,000 x g and discard the filtrate. Repeat for a total of two times.
- Add 50 μl of digestion buffer per sample to the retentate and pipette to a clean centrifuge tube.
Note: Steps B4 to B6 can be carried out while cell wall residue is resuspended.
- Pipette 50 μl of the enzyme solution obtained in step B6 to each of the resuspended cell wall samples from step B3.
- Incubate overnight in an orbital shaker at 37 °C and 150 rpm.
- The next day wash one centrifugal filter per sample by adding 500 μl of water, centrifuging for 3 min at 14,000 x g and removing the filtrate. Repeat this process for a total of 3 times.
Note: This step is necessary to remove traces of a polymer, which will otherwise interfere with the MALDI-TOF analysis.
- Centrifuge the cell wall digestion for 5 min at 18,000 x g.
- Pipette the supernatant to a pre-washed centrifugal filter. Centrifuge for 10 min at 14,000 x g.
- Discard the filter and dry the filtrate on a Speed-Vac.
Note: Some residue is usually visible at the bottom of the tube.
- Store at -20 °C.
- Add 1 ml of 100 mM NaCl to dried wall residue and resuspend in an ultrasonic bath for at least 20 min. Vortex the sample for 5 sec several times during resuspension. Centrifuge for 3 min at 18,000 x g and discard the supernatant.
- Extraction of non-accessible xyloglucan
- Wash the cell wall residue from the accessible xyloglucan extraction (precipitate from step B10) by adding 1 ml of water, centrifuging for 5 min at 18,000 x g and discarding the supernatant. Repeat for a total of two times.
- Add 900 μl of 17% NaOH (w/v) and incubate overnight in an orbital shaker at 37 °C.
- Add 300 μl of acetic acid and centrifuge for 5 min at 18,000 x g.
- Pipette 500 μl of the supernatant onto a centrifugal filter, centrifuge at 14,000 x g for 10-15 min and discard the filtrate. Add additional supernatant and repeat until all of it has been filtered.
- Add 500 μl of digestion buffer to the filter, centrifuge at 14,000 x g for 15-20 min and discard the filtrate. Repeat for a total of two times.
- Add 350 μl of digestion buffer to the filter and pipette the retentate to a new microcentrifuge tube.
- Follow the procedure for extraction of enzyme-accessible xyloglucan starting in step B4.
- Wash the cell wall residue from the accessible xyloglucan extraction (precipitate from step B10) by adding 1 ml of water, centrifuging for 5 min at 18,000 x g and discarding the supernatant. Repeat for a total of two times.
- MALDI-TOF analysis
- Resuspend dried xyloglucan digestion (step B13) in 20 μl of 10 mM NaCl by repeated pipetting. Centrifuge at 18,000 x g for 2 min.
- Pipette 2 μl from the top of the solution into a clean 200 μl PCR tube and add 6 μl of SDHB solution (see Recipes). Pipette 2 μl of the mixture onto the MALDI target plate.
- To prepare a calibration spot mix 2 μl of a 2 mM solution of xyloglucan oligosaccharides (Megazyme) with 6 μl of SDHB solution. Pipette 2 μl of the mixture onto the MALDI target plate.
- Air dry at room temperature for approximately 30 min.
Note: A dissecting microscope can be used to check the samples. Some crystals should be visible on the plate (Figure 2).
Figure 2. Examples of crystallization on the MALDI plate. Although the sample on the left is cleaner both produced usable spectra. Bars = 1 mm.
- Load the target plate onto a MALDI-TOF/TOF mass spectrometer.
- Operate the mass spectrometer in reflectron and positive mode, at an accelerating voltage of 25 kV with a PIE (Pulsed Ion Extraction) of 10 nsec. Set matrix suppression at 450 Da. Average a total of 600 laser shots delivered in 3 sets of 200 shots (at 100 Hz) to 3 different locations.
- Calibrate the mass range using the calibration spot. The three main peaks correspond to the following sodium adducts: XXXG (C39H66033Na1, 1085.3384 Da), XXLG/XLXG (C45H76038Na1, 1247.3912) and XLLG (C51H86043Na1, 1409.4440). These abbreviations correspond to the standard xyloglucan oligosaccharide nomenclature (Tuomivaara et al., 2015).
- Compile mass spectra for each sample by plotting the mass over charge (m/z) ratio (x-axis) of all detected ion species against their measured intensities (y-axis) using Flex Analysis Version 3.0 (Bruker).
- Resuspend dried xyloglucan digestion (step B13) in 20 μl of 10 mM NaCl by repeated pipetting. Centrifuge at 18,000 x g for 2 min.
Data analysis
- Using Flex Analysis label peaks using SNAP (Sophisticated Numerical Annotation Procedure) with signal to noise threshold set at 3. The program will calculate the area of the peaks from the isotopic envelope for each m/z (Figure 3). Export the data to Excel.
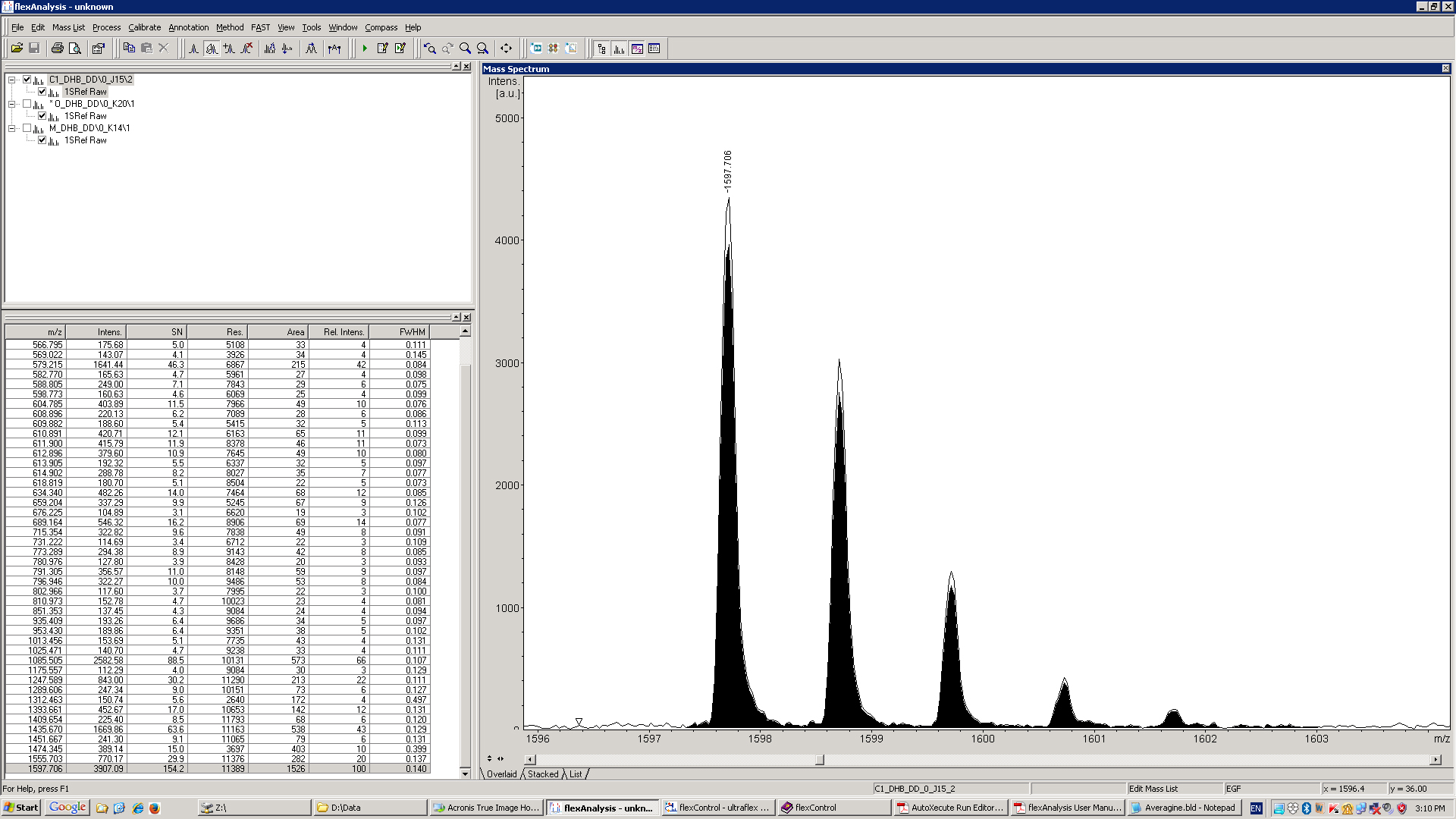
Figure 3. Screenshot of Flex Analysis showing a list of identified peaks with their areas, and a detail of the 1597 m/z peak
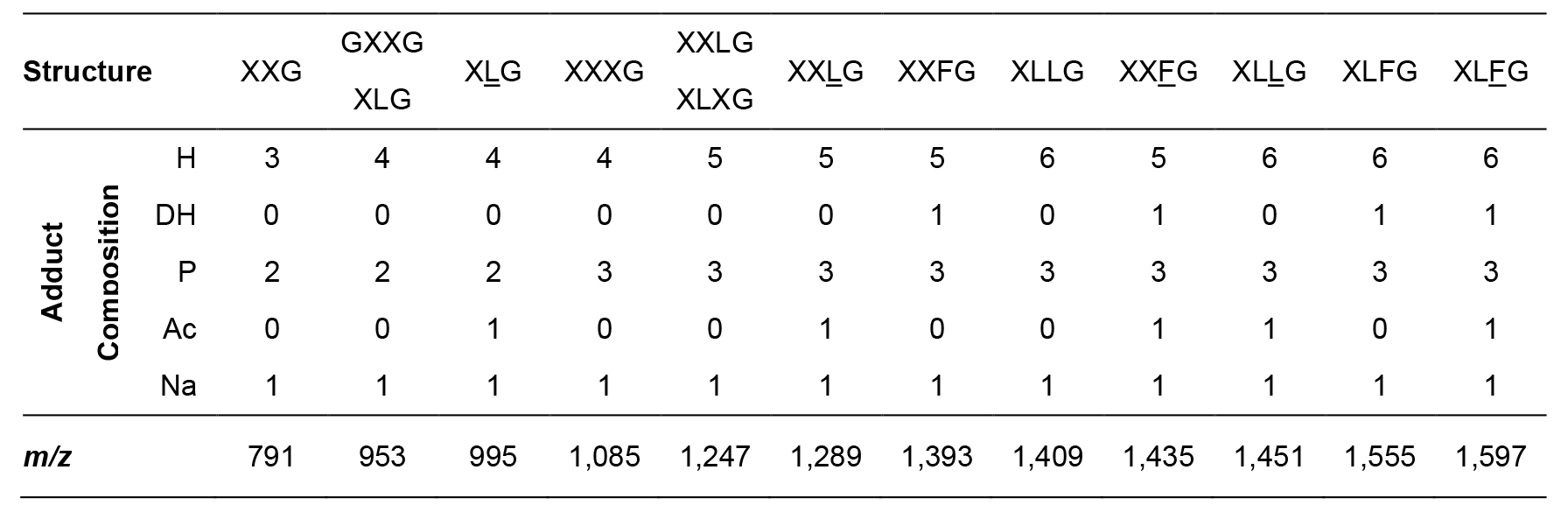
- Manually select the peaks with an m/z corresponding to xyloglucan fragments. Those present in wild-type xyloglucan are shown in Table 1. Sum the total area of the peaks and calculate the proportion of the total area that corresponds to each of the identified xyloglucan fragments. Average at least three independent extractions. Student’s t-test or other statistical methods can be used to compare the proportions of xyloglucan fragments in different genotypes. Extraction of non-accessible xyloglucan results in loss of acetyl groups and only non-acetylated fragments will be observed. Changes that are more evident in accessible xyloglucan, compared to non-accessible xyloglucan, are likely caused by xyloglucan metabolism in the wall.
Table 1. Structure and m/z values of xyloglucan fragments present in wild-type Columbia leaves. Structure abbreviations correspond to the standard xyloglucan oligosaccharide nomenclature, with acetylation indicated by underlined symbols (Tuomivaara et al., 2015). Adduct composition is indicated as the number of hexoses (H), deoxyhexoses (DH), pentoses (P), acetyl groups (Ac) and sodium atoms (Na). The m/z values of other oligosaccharide adducts can be calculated using the following formula: 162 x H + 146 x DH + 132 x P + 42 x Ac + 41.
Notes
An internal standard, such as malto-oligosaccharides or cello-oligosaccharides can be added to the SDHB solution for absolute quantification. Washes with water before extraction will remove a small amount of xyloglucan (Günl and Pauly, 2011). The endoglucanase used in this protocol appears to cut in front of every single unsubstituted glucose. Other endoglucanase with different specificities can produce a different set of fragments when analyzing unusual xyloglucan (Günl et al., 2011). This method cannot separate fragments of identical mass, such as XLXG and XXLG, although in some cases MALDI-TOF/TOF can be used on the same samples to identify the fragments (Sampedro et al., 2010; Sampedro et al., 2012; Sampedro et al., 2017).
Recipes
- Digestion buffer
36.6 μl acetic acid
29 μl pyridine
20 mg thimerosal
100 ml of water
Store at 4 °C
- SDHB matrix
9 mg ml-1 2,5-dihydroxybenzoic acid
1 mg ml-1 2-hydroxy-5-methoxybenzoic
70% acetonitrile
30% water
Store at -20 °C for one month
Acknowledgments
This protocol was briefly described in Sampedro et al. (2017). This work was supported by the Ministerio de Economía y Competividad (grant No. BIO2012-40032-C03-01) and the Xunta de Galicia (grant No. PGIDIT10PXIB200305PR).
References
- Günl, M., Gille, S. and Pauly, M. (2010). OLIgo mass profiling (OLIMP) of extracellular polysaccharides. J Vis Exp (40).
- Günl, M., Neumetzler, L., Kraemer, F., de Souza, A., Schultink, A., Pena, M., York, W. S. and Pauly, M. (2011). AXY8 encodes an α-fucosidase, underscoring the importance of apoplastic metabolism on the fine structure of Arabidopsis cell wall polysaccharides. Plant Cell 23(11): 4025-4040.
- Günl, M. and Pauly, M. (2011). AXY3 encodes a α-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 233(4): 707-719.
- Lerouxel, O., Choo, T. S., Seveno, M., Usadel, B., Faye, L., Lerouge, P. and Pauly, M. (2002). Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiol 130(4): 1754-1763.
- Park, Y. B. and Cosgrove, D. J. (2015). Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol 56(2): 180-194.
- Pauly, M., Albersheim, P., Darvill, A. and York, W. S. (1999). Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J 20(6): 629-639.
- Sampedro, J., Gianzo, C., Iglesias, N., Guitian, E., Revilla, G. and Zarra, I. (2012). AtBGAL10 is the main xyloglucan β-galactosidase in Arabidopsis, and its absence results in unusual xyloglucan subunits and growth defects. Plant Physiol 158(3): 1146-1157.
- Sampedro, J., Pardo, B., Gianzo, C., Guitian, E., Revilla, G. and Zarra, I. (2010). Lack of α-xylosidase activity in Arabidopsis alters xyloglucan composition and results in growth defects. Plant Physiol 154(3): 1105-1115.
- Sampedro, J., Valdivia, E. R., Fraga, P., Iglesias, N., Revilla, G. and Zarra, I. (2017). Soluble and membrane-bound β-glucosidases are involved in trimming the xyloglucan backbone. Plant Physiol 173(2): 1017-1030.
- Tuomivaara, S. T., Yaoi, K., O'Neill, M. A. and York, W. S. (2015). Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr Res 402: 56-66.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sampedro, J., Gianzo, C., Guitián, E., Revilla, G. and Zarra, I. (2017). Analysis of Xyloglucan Composition in Arabidopsis Leaves. Bio-protocol 7(19): e2569. DOI: 10.21769/BioProtoc.2569.
- Sampedro, J., Valdivia, E. R., Fraga, P., Iglesias, N., Revilla, G. and Zarra, I. (2017). Soluble and membrane-bound β-glucosidases are involved in trimming the xyloglucan backbone. Plant Physiol 173(2): 1017-1030.
Category
Plant Science > Plant biochemistry > Carbohydrate
Biochemistry > Carbohydrate > Xyloglucan
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










