- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Phagocytosis Assay of Necroptotic Cells by Cardiac Myofibroblasts
Published: Vol 7, Iss 18, Sep 20, 2017 DOI: 10.21769/BioProtoc.2552 Views: 7994
Reviewed by: Jia LiSuprabhat MukherjeeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3551 Views

Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3472 Views

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views
Abstract
In myocardial infarction (MI), a plenty of cardiomyocytes undergo necrosis and necroptosis due to the lack of oxygen and nutrients. The dead cardiomyocytes are promptly engulfed by phagocytes. When the dead cells are not engulfed, the noxious contents of the cells are released outside, and thus, induce inflammation, and obstruct the function of organs. Therefore, phagocytosis is crucial for maintaining homeostasis of organs. Herein, we describe a protocol of an in vitro phagocytosis assay of necroptotic cells.
Keywords: Phagocytosis assayBackground
Previously, necrotic and necroptotic cells were believed to be eliminated only by cardiac macrophages in failed hearts. However, we found that cardiac myofibroblasts, which are responsible for tissue fibrosis, engulf dead cells after an MI (Nakaya et al., 2017). Herein, we provide a detailed protocol for an in vitro phagocytosis assay of necroptotic cells, employing L929 cells that undergo necroptosis by TNF-α stimulation in a caspase-3 inhibitor, Z-VAD-FMK.
Materials and Reagents
- Pipette tips, 1,000 µl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2179-HR )
- Pipette tips, 200 µl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2069-HR )
- Pipette tips, 20 µl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2149P-HR )
- Pipette tips, 10 µl (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 2140-HR )
- Surgical tape (3M, catalog number: 1527-0 )
- 8-0 braided silk (NATSUME SEISAKUSHO, catalog number: M6-80B2 )
- 5-0 braided silk (NATSUME SEISAKUSHO, catalog number: ER12-50B1 )
- 10 ml syringe (TERUMO, catalog number: SS-10ESZ )
- 23-gauge needle (TERUMO, catalog number: NN-2332R )
- 50 ml tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339652 )
- 6 cm dish (Corning, catalog number: 430589 )
- Surgical lancet (Akiyama Medical MFG, catalog number: FB10 )
- 70 µm EASYstrainerTM (Greiner Bio One International, catalog number: 542070 )
- 10-cm non-treated dish (Corning, catalog number: 430591 )
- 15 ml tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 339650 )
- 8-well slide chamber (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 154534 )
- Cover glass (Matsunami Glass, catalog number: C024601 )
- 0.22 µm Minisart® filter (Sartorius, catalog number: 16534-K )
- Wild type C57BL/6JJmsSlc mouse (Japan SLC)
- L929 cells (National Institutes of Biomedical Innovation, Health and Nutrition, Japanese Collection of Research Bioresources Cell Bank, catalog number: JCRB9003 )
- Pentobarbital (Somnopentyl) (Kyoritsu Seiyaku, catalog number: SOM02-YA1312 )
- Phosphate buffered saline (PBS) (NACALAI TESQUE, catalog number: 14249-95 )
- Red blood cell (RBC) lysis buffer (Roche Diagnostics, catalog number: 11814389001 )
- Trypsin/Ethylenediaminetetraacetic acid (EDTA) (NACALAI TESQUE, catalog number: 35554-64 )
- Paraformaldehyde (PFA) (NACALAI TESQUE, catalog number: 26126-25 )
- 4’,6-Diamidino-2-phenylindole (DAPI) (Dojindo, catalog number: 340-07971 )
- FluorSaveTM (Merck, catalog number: 345789 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153-100G )
- Trypsin (Sigma-Aldrich, catalog number: T4799-5G )
- Collagenase A (Roche Diagnostics, catalog number: 10103586001 )
- Serum-free DMEM (NACALAI TESQUE, catalog number: 08458-16 )
- Penicillin streptomycin (NACALAI TESQUE, catalog number: 09367-34 )
- Dimethyl sulphoxide (DMSO) (Sigma-Aldrich, catalog number: D2650 )
- CellTrackerTM Green 5-chloromethylfluorescein diacetate (CMFDA) dye (Thermo Fisher Scientific, InvitrogenTM, catalog number: C7025 )
- Z-VAD-FMK (Z-Val-Aal-Asp(OMe)-CH2F) (PEPTIDE INSTITUTE, catalog number: 3188-v )
- hTNF-α (PeproTech, catalog number: 300-01A )
- Poly-L-lysine solution (Sigma-Aldrich, catalog number: P4707 )
- Water (NACALAI TESQUE, catalog number: 06442-95 )
- Fatal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- Collagenase A solution (see Recipes)
- Culture medium (see Recipes)
- 10 mM CMFDA dye (see Recipes)
- 10 mM Z-VAD-FMK (see Recipes)
- 10 µg/ml hTNF-α solution (see Recipes)
- 8-well slide chamber coated with poly-L-lysine (see Recipes)
Equipment
- Pipettes 1,000 µl (Gilson, catalog number: F123602 )
- Pipettes 200 µl (Gilson, catalog number: F123601 )
- Pipettes 20 µl (Gilson, catalog number: F123600 )
- Pipettes 2 µl (Gilson, catalog number: F144801 )
- Respirator (Shinano Manufacturing, catalog number: SN-480-7X2T )
- Optical microscope (Olympus, model: SZX7 )
- Surgical tools such as tweezers (tools can be purchased from NATSUME SEISAKUSHO and MEISTER)
- Scissors (NATSUME SEISAKUSHO, catalog number: B-12 )
- Clean bench (Panasonic Healthcare, model: MCV-B131S )
- Water bath (TAITEC, model: Personal-11 )
- Centrifuge (TOMY SEIKO, model: LC-200 )
- CO2 incubator (SANYO, model: MCO-18AIC )
- Fluorescence microscope (KEYENCE, model: BZ-9000 )
Software
- BZ-II image analysis application (KEYENCE CORPORATION)
Procedure
Procedures A and B are also applicable to the assay to determine phagocytosis of apoptotic cells by cardiac macrophages and Cardiac Myofibroblasts (Horii et al., 2017).
- Establishment of an MI mouse model by surgical operation
- Prepare an 8- to 10-week-old male wild type mouse of the strain, C57BL/6J.
- In order to anesthetize the mouse, administer pentobarbital (50 mg/kg) via an intraperitoneal injection.
- Fixate the mouse on its back with surgical tape.
- Perform artificial respiration by using a respirator (volume of air for respiration: 0.5 cc, respiratory frequency: 120 bpm).
- Under an optical microscope, surgically open the chest and expose the heart using sterilized surgical tools.
- Perform the permanent occlusion of the left coronary artery by using 8-0 braided silk (see Video 1).
- Close the chest by using 5-0 braided silk.
- After the operation, give the mouse the appropriate treatment, and keep it warm until recovery.
Video 1. How to establish an MI mouse model
- Prepare an 8- to 10-week-old male wild type mouse of the strain, C57BL/6J.
- Isolation of cardiac myofibroblasts from the MI mouse model
- Euthanize two mice after 3 days of the MI operation by administering pentobarbital (150 mg/kg) via an intraperitoneal injection.
- Open the chest by using sterilized scissors to expose the heart.
- Cut the right atrium and prick the left ventricle with a 10 ml syringe containing 10 ml of ice-cold PBS and mounted with a 23-gauge needle.
- Perfuse the heart with 10 ml of ice-cold PBS.
- Collect the heart, and remove the atria.
- Put the heart in a 50 ml tube containing 10 ml of ice-cold PBS for storage, while other mice are being sacrificed.
- Discard the supernatant and add 5 ml of ice-cold PBS.
- Transfer the heart along with PBS into a 6 cm dish from the 50 ml tube on a clean bench.
- Cut each heart into 15 small pieces using two surgical lancets, while keeping them on ice.
- Discard PBS.
- To wash the heart pieces, sprinkle 5 ml of PBS on them.
- Repeat steps B10-B11.
- Transfer the heart pieces into a 50 ml tube, and remove PBS.
- Add 4 ml of collagenase A solution (see Recipes) to the tube.
- Incubate the tube containing the mixture of the minced hearts and collagenase A solution in a water bath at 37 °C, while shaking at 120 rpm for 10 min.
- Discard supernatant which contains numerous hematopoietic cells.
- Repeat steps B14-B16 once again.
- Add 4 ml of collagenase A solution to the tube which now contains the residual material.
- Incubate the tube containing the mixture of the minced hearts and collagenase A solution in a water bath at 120 rpm and 37 °C for 10 min.
- Pass the supernatant, which contains isolated cells, through a 70 µm EASYstrainerTM into a new 50 ml tube.
- Centrifuge the collected cell suspension at 300 x g for 5 min at room temperature, and discard supernatant.
- Add 1 ml of the culture medium (see Recipes) to the cell pellet, and keep it on ice.
- Repeat steps B18-B22, eight times in total.
- After eight agitations, combine all the cell suspensions (1 ml x 8) into a new 50 ml tube.
- Centrifuge this cell suspension at 300 x g for 5 min, and discard the supernatant after centrifugation.
- Suspend the cell pellet in 1 ml of RBC lysis buffer, and incubate for 1 min at room temperature.
- Add 9 ml of the culture medium to the cell suspension.
- Centrifuge the cell suspension at 300 x g for 5 min, and discard the supernatant after centrifugation.
- Suspend the cell pellet in 10 ml of the culture medium.
- Plate this cell suspension on a 10-cm non-treated dish, and incubate overnight in a 5% CO2 incubator at 37 °C.
- After overnight incubation, discard the culture medium, and add 10 ml of fresh culture medium.
- Culture the isolated cardiac cells for more than 6 days.
Note: Myofibroblasts attach themselves to the plate; unattached contaminating cells, including hematopoietic cells, are removed by changing the culture medium. When the myofibroblasts reach pre-confluence, passage them. - One day before conducting the in vitro phagocytosis assay, discard the culture medium from the dish, and wash the dish twice with 10 ml of PBS.
- Add 1 ml of trypsin/EDTA, and incubate at 37 °C for 1 min.
- Add 9 ml of the culture medium, and transfer the cell suspension into a 15-ml tube.
- Centrifuge the cell suspension at 300 x g for 5 min, and discard the supernatant after centrifugation.
- Add 10 ml of the culture medium, and suspend the cell pellet.
- Count the cell number, and adjust it to 1 x 105 cells/ml by adding the culture medium.
- Add 200 µl of this cell suspension to an 8-well slide chamber coated with poly-L-lysine (2 x 104 cells/well, see Recipes).
- Incubate in a 5% CO2 incubator at 37 °C overnight.
- Euthanize two mice after 3 days of the MI operation by administering pentobarbital (150 mg/kg) via an intraperitoneal injection.
- Preparation of necroptotic L929 cells
- Seed 2.5 x 106 L929 cells on a 10 cm non-treated dish containing the culture medium.
- Incubate in a 5% CO2 incubator at 37 °C overnight.
- Aspirate the medium, and wash twice using 10 ml of serum-free DMEM.
- Add 10 ml of serum-free DMEM containing 10 µl of 10 mM CMFDA dye (see Recipes) (final concentration would be 10 µM).
- Incubate in a 5% CO2 incubator at 37 °C for 30 min.
- Aspirate the medium, and incubate on ice for 1 min.
- Wash 4 times using 10 ml of the culture medium.
- Add 10 ml of the culture medium containing 20 µl of 10 mM Z-VAD-FMK (see Recipes) (final concentration would be 20 µM).
- Incubate in a CO2 incubator for 2 h.
- Add 10 µl of 10 µg/ml hTNF-α solution (see Recipes) (final concentration is 10 ng/ml).
- Incubate in a 5% CO2 incubator at 37 °C for 4 h.
- Transfer the supernatant into a 50 ml tube.
Note: The supernatant contains necroptotic L929 cells. - Centrifuge the cell suspension at 300 x g for 5 min, and discard the supernatant after centrifugation.
- Add 10 ml of the culture medium.
- Repeat steps C13 and C14 thrice totally.
- Count the cell number.
- Adjust the cell number to 1 x 106 cells/ml with culture medium.
- Seed 2.5 x 106 L929 cells on a 10 cm non-treated dish containing the culture medium.
- Phagocytosis assay
- Prepare the cardiac myofibroblasts cultured with 200 µl of culture medium per well on the 8-well slide chamber in a 5% CO2 incubator at 37 °C (this slide was made in B40).
- Discard the supernatant.
- Add 200 µl of CMFDA-labeled necroptotic L929 cells at a concentration of 1 x 106 cells/ml (2 x 105 cells/well).
Note: The number of engulfed necroptotic cells is 10 times the number of phagocytes per well. - Incubate for 3 h at 37 °C in a 5% CO2 incubator.
- Discard the supernatant, and wash with 200 µl of PBS thrice.
Note: Unengulfed necroptotic cells are attached to phagocytes, and these cells are removed by washing with PBS. - To fix the cells, add 200 µl of 1% PFA/PBS solution, and incubate for 15 min at room temperature.
- Discard the supernatant, and wash with 200 µl of PBS.
- Discard the supernatant, and remove the chamber wall from the glass slide.
- Mount the slide with FluorSaveTM reagent containing 0.1% DAPI, and place a cover glass over the glass slide.
- Observe under phase contrast and fluorescence microscopes.
Note: Images are captured at 20x magnification by BZ-9000 . Obtain phase contrast images as well as the images captured by using GFP-BP and DAPI-BP filters.
- Prepare the cardiac myofibroblasts cultured with 200 µl of culture medium per well on the 8-well slide chamber in a 5% CO2 incubator at 37 °C (this slide was made in B40).
Data analysis
- Capture three types of images (phase contrast images as well as images obtained using GFP-BP and DAPI-BP filters) from 12-15 randomly selected fields (Figure 1).
- Merge the images using the BZ-II image analysis application.
- Count the number of the phagocytes and engulfed cells (CMFDA-positive and merged phagocytic cells).
Note: In the phase contrast images, unengulfed cells can be observed in the form of light phase and blurry images, whereas engulfed cells can be observed in the form of dark phase images. Do not consider these light phase cells as engulfed cells. - Calculate the phagocytosis index (Figure1).
Note: Phagocytosis index is defined as the number of engulfed cells per phagocyte. First, count the number of nuclei of myofibroblasts that are stained by DAPI (pink arrowheads) and then count the number of engulfed necroptotic cells labeled with CMFDA (yellow arrowheads). After the counting, phagocytosis index is calculated as the number of engulfed necroptotic cells divided by the number of nuclei of myofibroblasts. For example, when the number of nuclei of myofibroblasts is 11 and the number of engulfed necroptotic cells is 5, phagocytosis index is calculated as 5/11 = 0.45. Following the calculation, phagocytosis index is averaged over 12-15 randomly selected fields.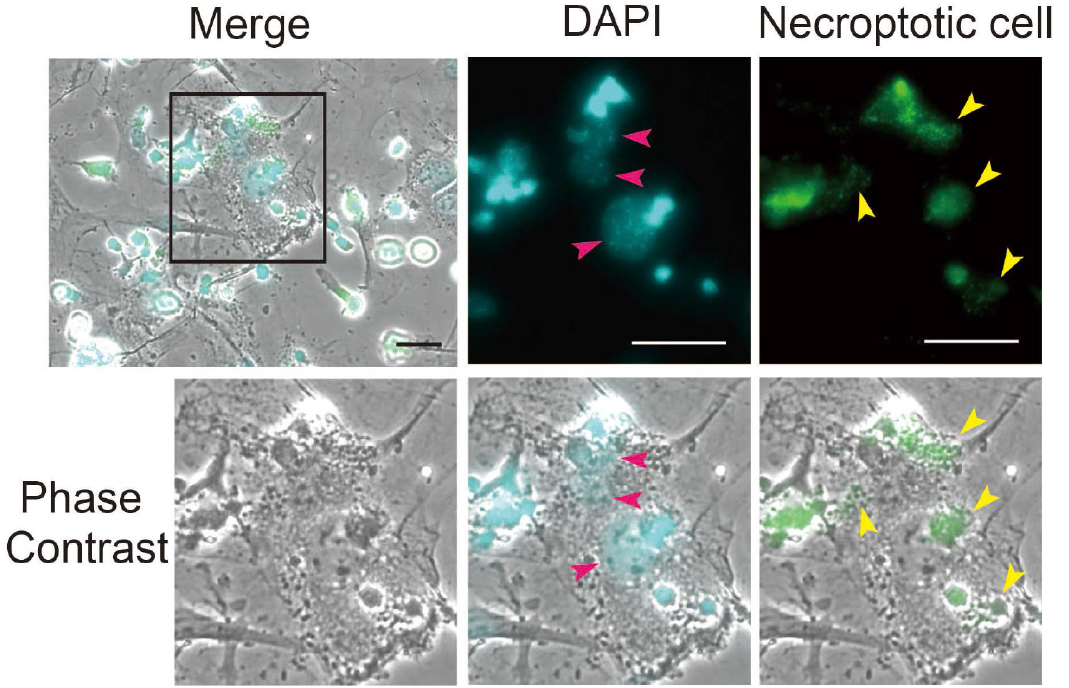
Figure 1. Cardiac myofibroblasts engulf the necroptotic cells. Cardiac myofibroblasts were isolated from the MI mouse models 3 days after operation. The necroptotic L929 cells are labeled with CMFDA (green). The pictures with arrowheads were rescaled to allow better visualization of phagocytosis. Count the number of phagocytes (pink arrowheads) and engulfed cells (yellow arrowheads), and calculate the phagocytic index. Scale bars = 50 µm.
Recipes
- Collagenase A solution
- Take 50 ml PBS to a 50 ml tube
- Add:
50 mg of BSA
50 mg of trypsin
50 mg of collagenase A - Mix well, and incubate at room temperature
- Filter the solution through a 0.22 µm Minisart® filter
- Take 50 ml PBS to a 50 ml tube
- Culture medium
- Remove 55 ml of serum-free DMEM from 500 ml of serum-free DMEM
- Add 50 ml of FBS
- Add 5 ml of penicillin-streptomycin
- Remove 55 ml of serum-free DMEM from 500 ml of serum-free DMEM
- 10 mM CMFDA dye
Add 10.76 µl of DMSO to 50 µg of CMFDA dye - 10 mM Z-VAD-FMK
Add 240 µl of DMSO to 1.1 mg of Z-VAD-FMK - 10 µg/ml hTNF-α solution
- Add 10 ml of sterile distilled water to a 15-ml tube
- Add 10 mg of BSA
- Filter the solution through a 0.22 µm Minisart® filter and make 0.1% BSA solution
- Add 100 µl of 0.1% BSA solution to 10 µg of hTNF-α and make 100 µg/ml of hTNF-α solution
- Add 90 µl of 0.1% BSA solution to 10 µl of 100 µg/ml of hTNF-α solution
- Add 10 ml of sterile distilled water to a 15-ml tube
- 8-well slide chamber coated with poly-L-lysine
- Add 200 µl of poly-L-lysine solution to the 8-well slide chamber
- Incubate in a CO2 incubator for 3 h
- Discard the poly-L-lysine solution
- Wash using PBS
- Add 200 µl of poly-L-lysine solution to the 8-well slide chamber
Acknowledgments
When using this protocol, please refer to M Nakaya et al. (2017) J Clin Invest. Funding was provided by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) [to M.N (17H03984)]; from Grant-in-Aid for Scientific Research on Innovative Areas (Homeostatic regulation by various types of cell death) from MEXT [to M.N (17H05510)]. All animal experiments were performed using approved protocols and in accordance with the guidelines of Kyushu University.
References
- Horii, Y., Matsuda, S., Watari, K., Nagasaka, A., Kurose, H. and Nakaya, M. (2017). An assay to determine phagocytosis of apoptotic cells by cardiac macrophages and cardiac myofibroblasts. Bio Protoc 7(18): e2553.
- Nakaya, M., Watari, K., Tajima, M., Nakaya, T., Matsuda, S., Ohara, H., Nishihara, H., Yamaguchi, H., Hashimoto, A., Nishida, M., Nagasaka, A., Horii, Y., Ono, H., Iribe, G., Inoue, R., Tsuda, M., Inoue, K., Tanaka, A., Kuroda, M., Nagata, S. and Kurose, H. (2017). Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest 127(1): 383-401.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Horii, Y., Matsuda, S., Watari, K., Nagasaka, A., Kurose, H. and Nakaya, M. (2017). Phagocytosis Assay of Necroptotic Cells by Cardiac Myofibroblasts. Bio-protocol 7(18): e2552. DOI: 10.21769/BioProtoc.2552.
Category
Immunology > Immune cell function > Macrophage
Immunology > Immune cell isolation > Macrophage
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









