- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Improved Oviduct Transfer Surgery for Genetically Modified Rat Production
Published: Vol 7, Iss 16, Aug 20, 2017 DOI: 10.21769/BioProtoc.2503 Views: 10086
Reviewed by: Jingli CaoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1830 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3773 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2637 Views
Abstract
Rat embryo transfer surgeries are becoming more common with targeted nucleases increasing the demand for rat models. This protocol details pre-surgical preparation, improved surgical techniques for placing embryos into the oviduct, and post-surgical care of rats to parturition. Direct application of mouse oviduct transfer protocols results in limited success in the rat. By combining techniques from several widely used protocols in the field, increased yield of live pups born to healthy dams is possible. This protocol is distinct from previously published protocols by the use of a modified anesthesia protocol (Smith et al., 2004), use of analgesia, the addition of peritoneal sutures (Filipiak and Saunders, 2006), incision location and number of transfers per animal (Krinke et al., 2000).
Keywords: RatBackground
The ability to reliably produce healthy pups after microinjection and embryo transfer surgery is critical to model creation and, in particular, the increased likelihood of creating multiple founder animals gives confidence in the phenotypes observed. Therefore, as birth rates were low relative to reported rates even with varied concentrations of injection solution, modifications were systematically made to the existing mouse embryo transfer protocol to better suit the rat.
Multiple publications describe transferring embryos to the oviducts of both horns of the bipartite uterus; however, this increases the length of time the animal is under anesthesia and requires either a midline incision and traversing the peritoneal cavity to reach the lateral reproductive tract, or creating two separate incisions (Krinke et al., 2000). These options are less than ideal since either will increase stress of the animal and thereby the likelihood that the pregnancy will be aborted. By creating a single lateral incision and administering analgesia both preoperatively and postoperatively the stress of the animal is minimized (Smith et al., 2004). The use of isoflurane over injectable anesthetic agents minimizes risk of toxicity (such as seen with tribromoethanol), injury from IP injection, and repeated dosing, all of which are associated with higher mortality rates following rodent surgery (Bernal et al., 2009).
The greatest improvements in litter number and size followed the addition of ampicillin and epinephrine to the procedure [62 born/298 transferred (20.8%) versus 91 born/248 transferred (36.7%) post addition of ampicillin and epinephrine; all projects]. Although the surgery is performed aseptically, ampicillin was shown as early as 1995 to optimize the number of pups born to rats (Waller, 1995) and use of epinephrine on the ovarian bursa reduced bleeding and thereby trauma to the animal, as well as reduced the length of time required to find the infundibulum. These modifications have been used individually in multiple reports; however, this is the first protocol to combine the most advantageous aspects of each protocol while refining procedures that may be detrimental (Krinke et al., 2000; Smith et al., 2004; Filipiak and Saunders, 2006).
Materials and Reagents
- Personal protective materials–hair net, gloves
- 4-0 black silk sutures (Kent Scientific, catalog number: INS701073 )
- 9 mm wound clips (BD, catalog number: 427631 )
- Kimwipes (KCWW, Kimberly-Clark, catalog number: 34155 )
- Insulin syringes (BD, catalog number: 329412 )
- Iodine swabs (PDI Healthcare, catalog number: S41350 )
- (Optional) Rodent mask diaphragms (Smiths Medical, Surgivet, catalog number: 32247B1 )
- Sterile surgical drape
- 500 ml filter system
- Fertilized one cell Sprague Dawley embryos (see Notes)
- 8-week old female Sprague Dawley recipient rats
- Vasectomized Sprague Dawley male rats
- Luteinizing hormone releasing hormone agonist (LHRHa) (Sigma-Aldrich, catalog number: L4513 )
- 70% ethanol
- Buprenorphine (Southern Anesthesia & Surgical, catalog number: 12496075705 )
- Carprofen (Zoetis Services, catalog number: 060062 )
- Ampicillin (Fisher Scientific, catalog number: BP1760-25 )
- 0.1% epinephrine (Acros Organics, catalog number: 204400010 )
- Sterile, nonmedicated ophthalmic ointment (Rugby Laboratories, catalog number: 301905 )
- Embryo tested water (Sigma-Aldrich, catalog number: W1503 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P9791 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: M1880 )
- Glucose (Sigma-Aldrich, catalog number: 158968 )
- Penicillin (Sigma-Aldrich, catalog number: P7794 )
- Streptomycin (Sigma-Aldrich, catalog number: S1277 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
- Sodium pyruvate (Sigma-Aldrich, catalog number: P4562 )
- EDTA (Sigma-Aldrich, catalog number: 03609 )
- L-Glutamine (Sigma-Aldrich, catalog number: G8540 )
- Sodium lactate (Sigma-Aldrich, catalog number: L7900 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Phenol red (Sigma-Aldrich, catalog number: P0290 )
- Isoflurane (AMERISOURCE BERGEN, catalog number: 10103618 )
- LHRHa solution (see Recipes)
- KSOM medium (see Recipes) (Cold Spring Harbor, 2006)
Equipment
- Biosafety cabinet
- pH meter
- Mouth pipet (Fisher Scientific, catalog number: NC9048719 )
Manufacturer: BIOTECH, model: MP001Y. - Glass pipettes (Fisher Scientific, catalog number: 13-678-20C )
- Flame source to pull pipettes
- Personal protective equipment–clean lab coat
- 9 mm wound clip applier (BD, catalog number: 427630 )
- Microscope (Leica Microsystems, model: Leica S8 APO )
- Fine forceps (Fine Science Tools, catalog number: 11251-10 )
- Spring scissors (Roboz Surgical Instrument, catalog number: RS-5650 )
- Large scissors (Roboz Surgical Instrument, catalog number: RS-5910 )
- Grip forceps (Roboz Surgical Instrument, catalog number: RS-8100 )
- Micro clip (Roboz Surgical Instrument, catalog number: RS-5420 )
- Versi-Dry surface protectors (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 74000-00 )
- 37 °C warming plate (C & A Scientific, Premiere, catalog number: XH-2002 )
- Animal clippers (Oster, catalog number: 078005-301-003 )
- Bead sterilizer (CellPoint Scientific, catalog number: 5-1450 )
- Anesthesia machine (Smiths Medical, Surgivet, catalog number: WWV9000 )
- Rodent anesthesia circuit set (Smiths Medical, Surgivet, catalog number: V7103 )
- Large rubber bands
Procedure
- Between 11:00 AM and 1:00 PM five days before surgery, 0.2 ml of 200 μg/ml LHRHa solution (see Recipes) is injected into the intraperitoneal cavity of the female recipient rats to synchronize estrus.
- At noon one day prior to surgery the female recipient rats are mated with vasectomized males.
- The following morning females are checked for the presence of a vaginal copulation plug as proof of mating. Females with vaginal plugs are used in the procedure and negative females are placed back into the colony for two weeks before reuse.
- The surgeon should wear a clean lab coat, hair net and gloves. A disinfected area is prepared by wiping the base of the microscope with 70% ethanol and placement of a clean surface protector. Embryos to be transferred are produced and collected as previously described (Filipiak and Saunders, 2006) and are incubated in KSOM (see Recipes) at 37 °C up to 24 h before the transfer.
- Instruments are autoclaved before the first surgery and sterilized in between up to four additional surgeries. Between surgeries, wipe instruments with sterile saline then insert the tip of each instrument into a 250 °C bead sterilizer for 15 sec. Allow to cool a minimum of one minute on a sterile Kimwipe before use.
- Anesthesia is induced in the recipient rat typically within 3-5 min of placement in a chamber with 1.0-1.5 liters per minute (L/min) of 5% isoflurane with oxygen as a carrier gas. Anesthesia is maintained during surgery with 3% isoflurane by placement of the nose of the preanesthetized rat in a nose cone (Figure 1). A diaphragm can be created using a glove and rubber band and cutting a small hole to fit snugly around the nose of the anesthetized rat (see Notes).
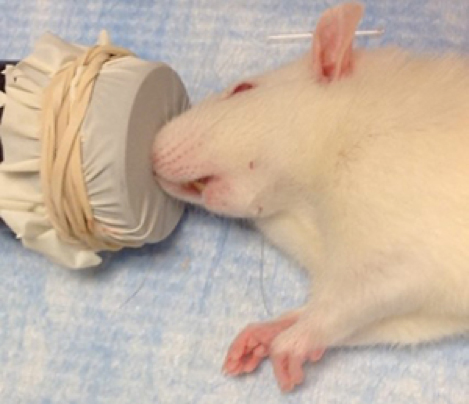
Figure 1. Anesthetized rat receiving isoflurane through tight fitting nose cone - Apply ophthalmic ointment to the eyes by squeezing a thin layer of ointment onto the eye without contaminating the tube by touching the eye directly.
- Separate subcutaneous injections of buprenorphine (0.05 mg/kg), carprofen (5 mg/kg) and 0.5 ml of ampicillin (100 mg/ml) also take place immediately prior to surgery.
- Surgical depth of anesthesia is ensured first by observation of a depressed respiratory rate, then by repeated absence of tail- or foot-pinch reflex.
- The lower back of the recipient rat is shaved above the left uterine horn, and placed on a sterile tissue on the stage of the microscope.
- The shaved area is blotted with iodine and wiped with 70% ethanol-soaked tissue to prepare the surgical field and remove excess hair particles caused by shaving (Figure 2). This is repeated twice.
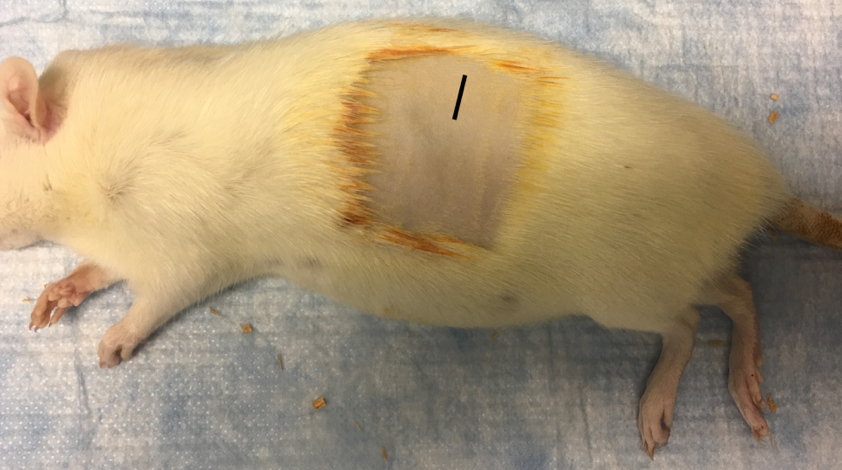
Figure 2. Rat fully prepared for surgery after shaving and sterilization. Incision location indicated by black line. - A sterile drape is placed over the animal.
- A single incision about 1 cm in length in a dorsal to ventral direction is made in the skin using grip forceps and large scissors at the level of the last rib (Figure 3).
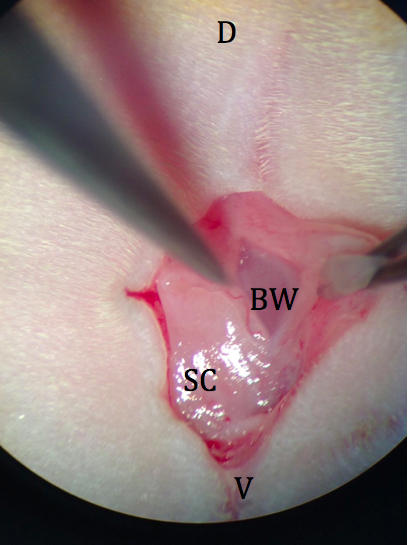
Figure 3. Initial incision through skin and subcutaneous fat. D: dorsal; V: ventral; SC: subcutaneous fat; BW: body wall. - The body wall is picked up with fine forceps and a small incision is made with spring scissors (avoiding blood vessels) just over the left ovarian fat pad (Figure 4).
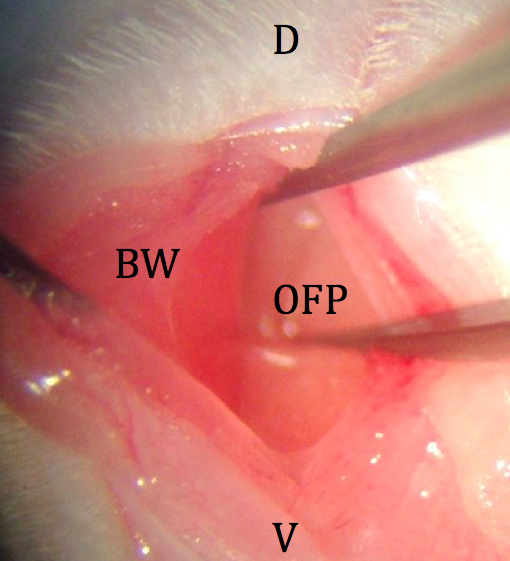
Figure 4. Incision through body wall. D: dorsal; V: ventral; BW: body wall; OFP: ovarian fat pad. - The ovarian fat pad is grasped with fine forceps, and the distal portion of the reproductive tract is gently retracted from the abdominal cavity. The fat pad is held in place by an attached micro clip with a sterile piece of tightly rolled tissue under the uterus (Figure 5).
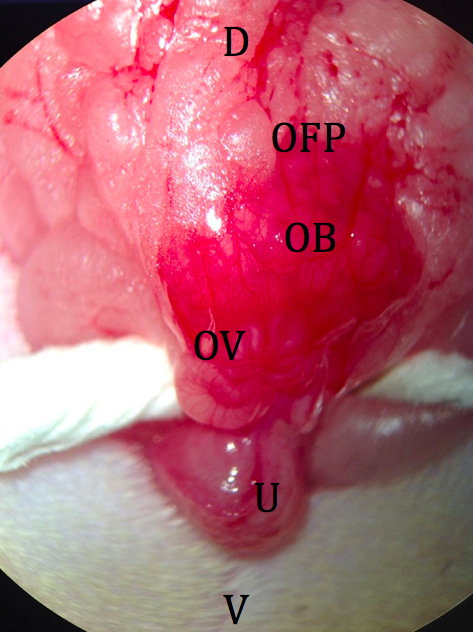
Figure 5. Externalized ovary and fat pad. D: dorsal; V: ventral; OFP: ovarian fat pad; OB: ovarian bursa; OV: oviduct; U: uterus. - A minimally vascularized area of the ovarian bursa is manually torn with fine forceps, providing a route to the ostium of the oviduct (Figure 6).
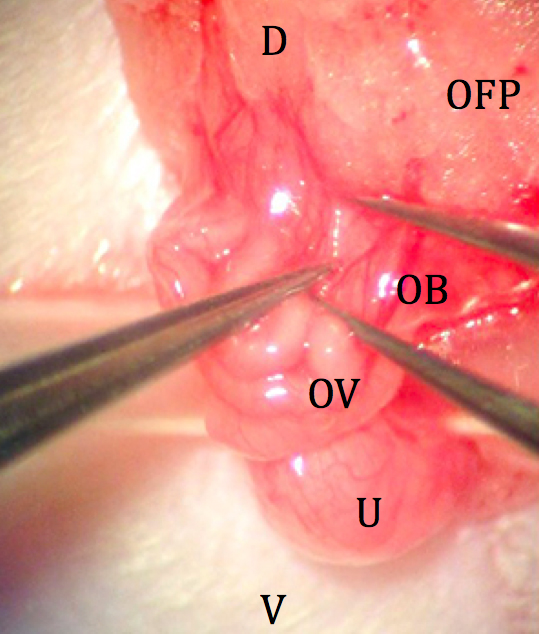
Figure 6. Location of minimally vascularized area of ovarian bursa. D: dorsal; V: ventral; OFP: ovarian fat pad; OB: ovarian bursa; OV: oviduct; U: uterus. - 1-2 drops of epinephrine (approximately 20 μl) are dripped onto the bursa from a syringe until bleeding ceases. This decreases the length of the surgery and expedites location of the ostium.
- The tip of the transfer mouth pipet is loaded with 15-20 embryos for implantation and inserted into the ostium of the oviduct after stabilization with fine forceps. The embryos are injected into the oviduct by gentle pressure through the pipette (Figure 7).
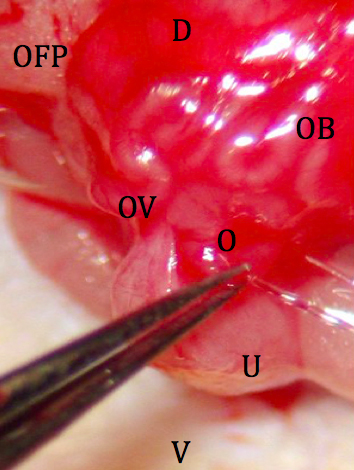
Figure 7. Placement of embryos into the reproductive tract. D: dorsal; V: ventral; OFP: ovarian fat pad; OB: ovarian bursa; OV: oviduct; U: uterus; O: ostium. - The reproductive tract is carefully replaced in the abdomen, and the abdomen is closed by 2 sutures in the body wall (Figure 8) followed by skin closure with 2 wound clips.
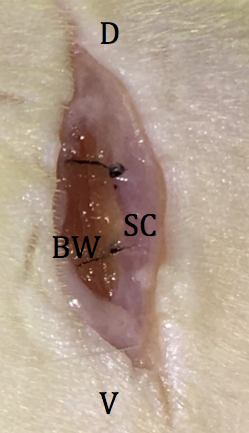
Figure 8. Location of sutures in peritoneal wall. D: dorsal; V: ventral; SC: subcutaneous fat; BW: body wall. - Following surgery, the recovering rat is placed inside a rat cage resting on a 37 °C warming plate until the animal is fully awake and upright, typically five to ten minutes (see Notes).
- The cage is returned to a cage rack in a rat room and provided subcutaneous injection 0.2 ml carprofen (5 mg/ml) after 24 h as post-surgical analgesia. Up to three females can be housed together.
- The rats are monitored daily to verify recovery from the procedure. Females are separated 20 days after the procedure to give birth in individual cages.
Data analysis
The research paper detailing generation of genetically modified rats using oviduct transfer surgery is available online (Lambert et al., 2016).
Notes
- Common strains of Rattus norvegicus include Sprague Dawley, Wistar, Dahl SS and F344. No sex determination of the embryos is necessary. Taconic and Charles River are recommended vendors.
- Two-cell embryos can also be transferred by this method.
- Between 5 and 25 embryos can be transferred to each female.
- An additional suture and/or wound clip can be used if the incision is larger than 1 centimeter.
- Rats have an excessive amount of fat compared to mice. Be careful to handle the fat pad without puncturing or tearing the tissue.
- Diaphragms can also be purchased with or without openings and cut to the desired size (Surgivet).
- Indications of a failed operation include depressed respiratory rate, labored breathing and/or gasping and lethargy.
Recipes
- LHRHa solution
Dissolve 1 mg of LHRHa powder in 5 ml sterile water for 200 μg/ml stock
Store at -20 °C in 1 ml aliquots - KSOM (Cold Spring Harbor, 2006)
500 ml embryo tested water
2.775 g NaCI
0.095 g KCl
0.025 g KH2PO4
0.025 g MgSO4·7H2O
0.02 g glucose
0.03 g penicillin
0.025 g streptomycin
1.05 g NaHCO3
0.01 g sodium pyruvate
0.002 g EDTA
0.073 g L-glutamine
0.935 g sodium lactate
0.125 g CaCl2·2H2O
0.5 g bovine serum albumin
50 μl phenol red
Combine reagents in 175 ml embryo tested water. Adjust volume to 500 ml. Using a 500 ml filter system, filter the solution in a biosafety cabinet. Check the pH and store remaining solution at 4 °C. Check the pH after incubation (pH should drop); ideal pH is 7.1-7.7
Acknowledgments
The University of Alabama at Birmingham Transgenic & Genetically Engineered Models facility (RAK) is supported by the National Institutes of Health [grant numbers P30 CA13148, P30 AR048311, P30 DK074038, P30 DK05336 and P60 DK079626] and by the UAB Cystic Fibrosis Research Center. All procedures were conducted with the approval of the IACUC and the UAB Animal Resources Program (ARP) and are in compliance with guidelines for the care and use of laboratory animals and rodent survival surgery (Bernal et al., 2009; Albus, 2012; Cunliff-Beamer, 1993; Waynforth, 1992). We acknowledge the work of Filipiak and Saunders which served as the baseline for adaptation.
References
- Albus, U. (2012). Guide for the care and use of laboratory animals (8th edition). Laboratory Animals 46(3): 267-268.
- Bernal, J., Baldwin, M., Gleason, T., Kuhlman, S., Moore, G. and Talcott, M. (2009). Guidelines for rodent survival surgery. J Invest Surg 22(6): 445-451.
- Cold Spring Harbor. KSOM. (2006). Cold Spring Harb Protoc.
- Cunliff-Beamer, T. L. (1993). Applying principles of aseptic surgery to rodents. AWIC Newsletter 4(2): 3-6.
- Filipiak, W. E. and Saunders, T. L. (2006). Advances in transgenic rat production. Transgenic Res 15(6): 673-686.
- Hankenson, F. C. (2013). Critical care management for laboratory mice and rats. CRC Press.
- Krinke, G. J., Bullock, G. R. and Bunton, T. (2000). The laboratory rat. Elsevier Science.
- Lambert, L. J., Challa, A. K., Niu, A., Zhou, L., Tucholski, J., Johnson, M. S., Nagy, T. R., Eberhardt, A. W., Estep, P. N., Kesterson, R. A. and Grams, J. M. (2016). Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology. Dis Model Mech 9(10): 1169-1179.
- Smith, J. C., Corbin, T. J., McCabe, J. G. and Bolon, B. (2004). Isoflurane with morphine is a suitable anaesthetic regimen for embryo transfer in the production of transgenic rats. Lab Anim 38(1): 38-43.
- Waller, S. J., Ho, M. Y. and Murphy, D. (1995). Production of transgenic rodents by microinjection of cloned DNA in fertilized one-cell eggs. In: Glover, D. M. and Hames, B. D. (Eds). DNA cloning. Vol. 4. Oxford University Press pp: 184-229.
- Waynforth, H. B. and Flecknell, P. A. (1992). Experimental and surgical technique in the rat. 2nd edition. Academic Press.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lambert, L. J., Johnson, L. W., Kennedy, D., Cadillac, J. and Kesterson, R. A. (2017). Improved Oviduct Transfer Surgery for Genetically Modified Rat Production. Bio-protocol 7(16): e2503. DOI: 10.21769/BioProtoc.2503.
Category
Cell Biology > Cell Transplantation > Embryo Transplant
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link