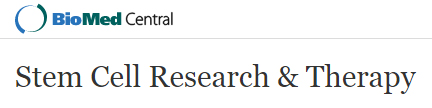- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Implantation of Human Peripheral Corneal Spheres into Cadaveric Human Corneal Tissue
Published: Vol 7, Iss 14, Jul 20, 2017 DOI: 10.21769/BioProtoc.2412 Views: 7705
Reviewed by: Federica PisanoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3789 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1255 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 144 Views
Abstract
Stem and progenitor cells isolated from human limbal tissue can be cultured in vitro as spheres. These spheres have potential for use as transplantable elements for the repopulation of corneal tissue (Mathan et al., 2016). Herein we describe the detailed protocol for the implantation of human corneal spheres into cadaveric human corneal tissue. This protocol describes the procedure for sphere formation and culture, preparation of tissue for sphere implantation, corneal limbus microsurgery and sphere implantation.
Keywords: Cell CultureBackground
Previous research has focused on isolation of limbal cells which were exclusively epithelial (limbal stem cell) or stromal (keratocyte progenitor cell) in order to decipher their individual roles in corneal homeostasis and wound repair. This protocol aims to isolate limbal cells by their functional ability to form spheres in culture and which by their very nature will include a diversity of cells both epithelial and stromal which contribute to the formation of the limbal niche. Subsequent to isolation of these spheres we are investigating their potential use in corneal restoration after implantation. Here we describe an in-vitro surgical protocol for the implantation of these spheres into human corneal tissue and the downstream analysis.
Materials and Reagents
- Scalpel blade (ProSciTech, profile 11) (Swann Morton, catalog number: LSB11 )
- 20 x 100 mm cell culture dish (Corning, Falcon®, catalog number: 353003 )
- Cotton buds (Cotton Tips double ended, Protec Solutions, catalog number: 941001690389 )
- Transfer pipette (3 ml) (Interlab, catalog number: KJ622-1A )
- 5 ml tubes (Techno Plas, catalog number: P5016UL )
- 40 µm strainer (Corning, Falcon®, catalog number: 352340 )
- Glass coverslips (covergalss #1 30 mm diam.) (PST, catalog number: G430 )
- 6-well tissue culture plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675 )
- 35 x 10 mm cell culture dish (Corning, Falcon®, catalog number: 353001 )
- Fresh and frozen human cadaveric donor corneoscleral tissue obtained post-surgery from the New Zealand National Eye Bank
- 70% ethanol (EMD Millipore, catalog number: 1009832500 )
- Phosphate buffered saline (PBS) sterile (Sigma-Aldrich, catalog number: P4417-100TAB )
- Phosphate buffered saline and 10% glycerol (AnalaR NORMAPUR) (VWR, catalog number: 24388.320 )
- Dispase (Thermo Fisher Scientific, GibcoTM, catalog number: 17105041 )
- Collagenase (Blend Type L) (Sigma-Aldrich, catalog number: C8176 )
- Hyaluronidase (Sigma-Aldrich, catalog number: H3506 )
- Neurobasal-A Medium (Thermo Fisher Scientific, GibcoTM, catalog number: 10888022 )
- Human Epidermal Growth Factor (EGF) (PeproTech, catalog number: AF-100-15 )
- Human Fibroblastic Growth Factor (FGF) Basic (PeproTech, catalog number: 100-18B )
- B-27 Supplement, 50x (Thermo Fisher Scientific, GibcoTM, catalog number: 12587010 )
- N-2 Supplement, 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 17502048 )
- GlutaMAX, 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- MEM with GlutaMAX (Minimum Essential Medium) (Thermo Fisher Scientific, GibcoTM, catalog number: 41090036 )
- Foetal bovine serum (New Zealand origin) (Thermo Fisher Scientific, GibcoTM, catalog number: 10091148 )
- Antibiotic/Antimycotic 100x (Anti/Anti) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240062 )
- Supplemented Neurobasal-A medium (see Recipes)
- Standard culture medium (see Recipes)
Equipment
- Tissue culture hood (Heal Force, model: HFsafe 1800 )
- Straight scissors (World Precision Instruments, catalog number: 500216 )
- Fine forceps (World Precision Instruments, catalog number: 14142 )
- Orbital shaker (Ratek Instruments, model: MM1 )
- Centrifuge (Sigma Laborzentrifugen, model: 3-15 )
- Tissue culture incubator (Thermo Fisher Scientific, Thermo ScientificTM, model: HereausTM HeraCell 150 )
- Laminar flow cabinet (Gelman, model: HLF 120 )
- Binocular Stereo microscope (Carl Zeiss, catalog number: 474110-9904 )
- Biological safety cabinet (Email Air Handling, catalog number: 1687-2340-618-3 )
- 2-20 µl Eppendorf® Pipette (Eppendorf, catalog number: 3120000038 )
- Feather MicroScalpel (pfm medical, catalog number: 200300715 )
- Crescent Bevel Up Ophthalmic Knife 2.3 mm (MANI, catalog number: MCU26 )
- NIKON Digital sight DS-UI camera (Nikon Instruments, model: DS-UI )
- Leica DMIL inverted contrasting microscope (Leica Microsystems, model: Leica DMIL )
- Leica DM-RA upright fluorescence microscope (Leica Microsystems, model: Leica DM-RA )
Software
- NIS-Elements Br Microscope Imaging Software version 3.0
Procedure
Note: This procedure has been described in previous work by our laboratory (Yoon et al., 2013), the following section however provides additional information on the process and describes the technique in a protocoled format.
- Sphere formation and culture (Video 1)
Video 1. Corneoscleral tissue processing for Sphere Forming Assay. Corneoscleral tissue is rubbed with a cotton bud on the posterior side to remove endothelial cells, scraped on the anterior side to remove epithelial cells and washed in PBS. The corneal/limbal region is excised and cut into small pieces prior to enzymatic treatment for limbal cell isolation.- Using sterile technique in a sterile tissue culture hood, prepare 1 container of 70% ethanol and 1 container of PBS for tools and 1 container of PBS for washing. Place straight scissors, scalpel blade and forceps in 70% ethanol for 10 min.
- Remove the above equipment from 70% ethanol and rinse briefly in sterile PBS to remove residual ethanol.
- Using forceps, place corneoscleral tissue into a 100 mm sterile Petri dish (the corneoscleral rim [Figure 1A] has an approximate 2-2.5 mm wide ring of corneal/limbal tissue, which when excised has an approximate wet weight of 55-57 mg).
- Using sterile cotton buds, rub the posterior surface of the corneal tissue to remove endothelial cells (Figure 1B).
- Using a crescent bevel up ophthalmic knife, gently scrape the anterior surface of the tissue to remove epithelial cells (Figure 1C).
- Using a sterile transfer pipette, wash tissue with PBS. Repeat epithelial scraping and washes until PBS washes run clear of cellular debris (Figure 1D).
- Excise the limbal region and exclude as much of the sclera as possible (Figure 1E). Rinse the excised limbus, mince the tissue using scissors (Figure 1F) and place into sterile 5 ml tubes.
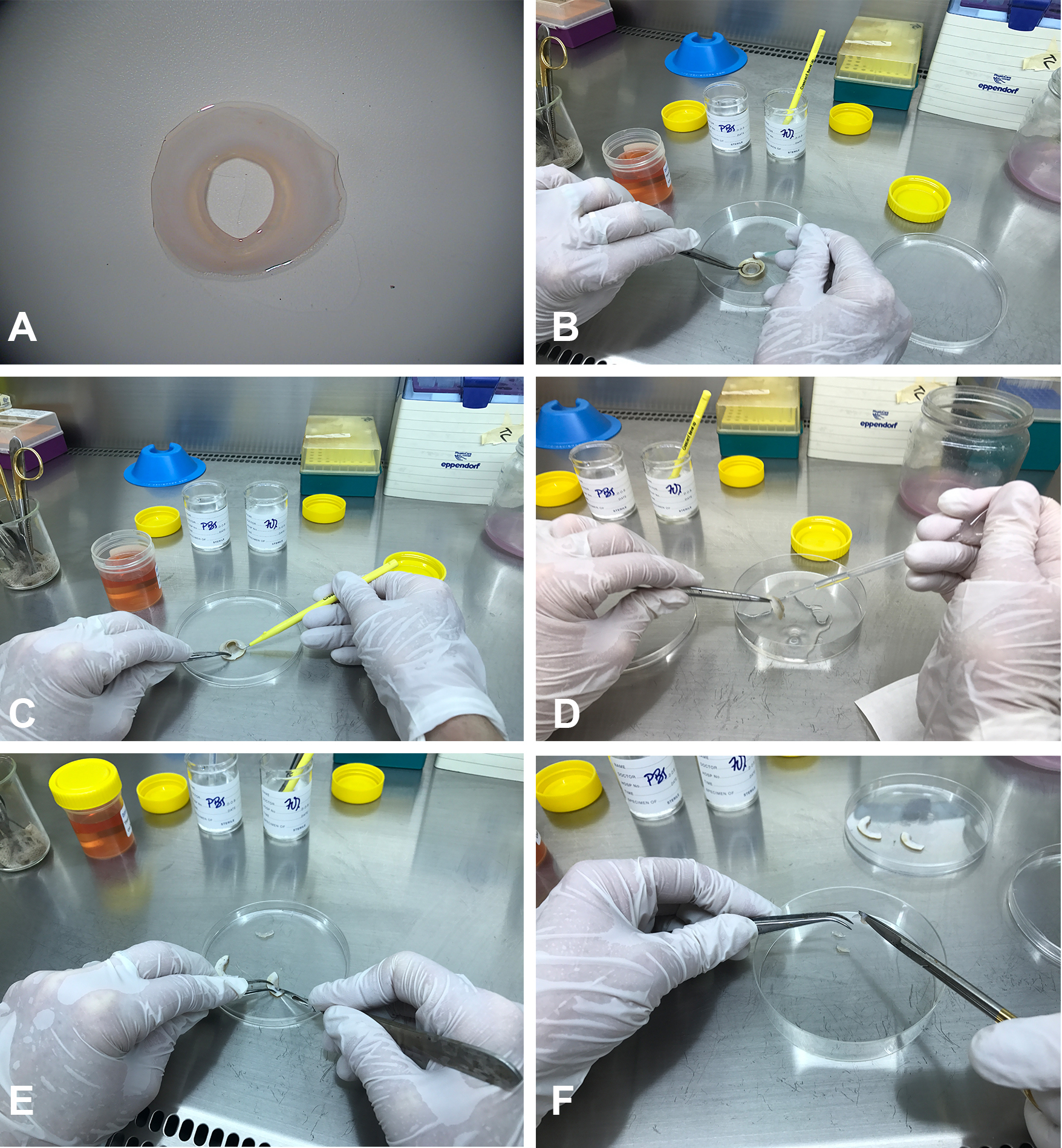
Figure 1. Corneoscleral tissue processing for Sphere Forming Assay. Corneoscleral tissue (A) is rubbed with a cotton bud on the posterior side to remove endothelial cells (B), scraped on the anterior side to remove epithelial cells (C) and washed in PBS (D). The corneal/limbal region is excised (E) and cut into small pieces (F) prior to enzymatic treatment for limbal cell isolation. - Incubate in 1.2 U/ml Dispase for 40 min at 37 °C.
- Incubate in 2 mg/ml collagenase and 0.5 mg/ml hyaluronidase in MEM with Anti/Anti overnight at 37 °C on an orbital shaker.
- Strain the solution and cells using a 40 µm strainer to remove undigested material.
- Centrifuge filtrate for 7 min at 405 x g and wash cell pellet with PBS.
- Suspend cells in 2 ml supplemented Neurobasal-A medium.
- Prepare sterile glass coverslips in 6-well tissue culture plates.
- Seed the 2 ml suspension evenly into wells.
- Culture cells in a humidified incubator at 37 °C in 5% CO2.
- Carefully change 50% of medium twice weekly pipetting from the surface to avoid removing cells or newly forming spheres.
- Cells often become adherent to glass coverslips and aggregate into sphere like structures (Figure 2).
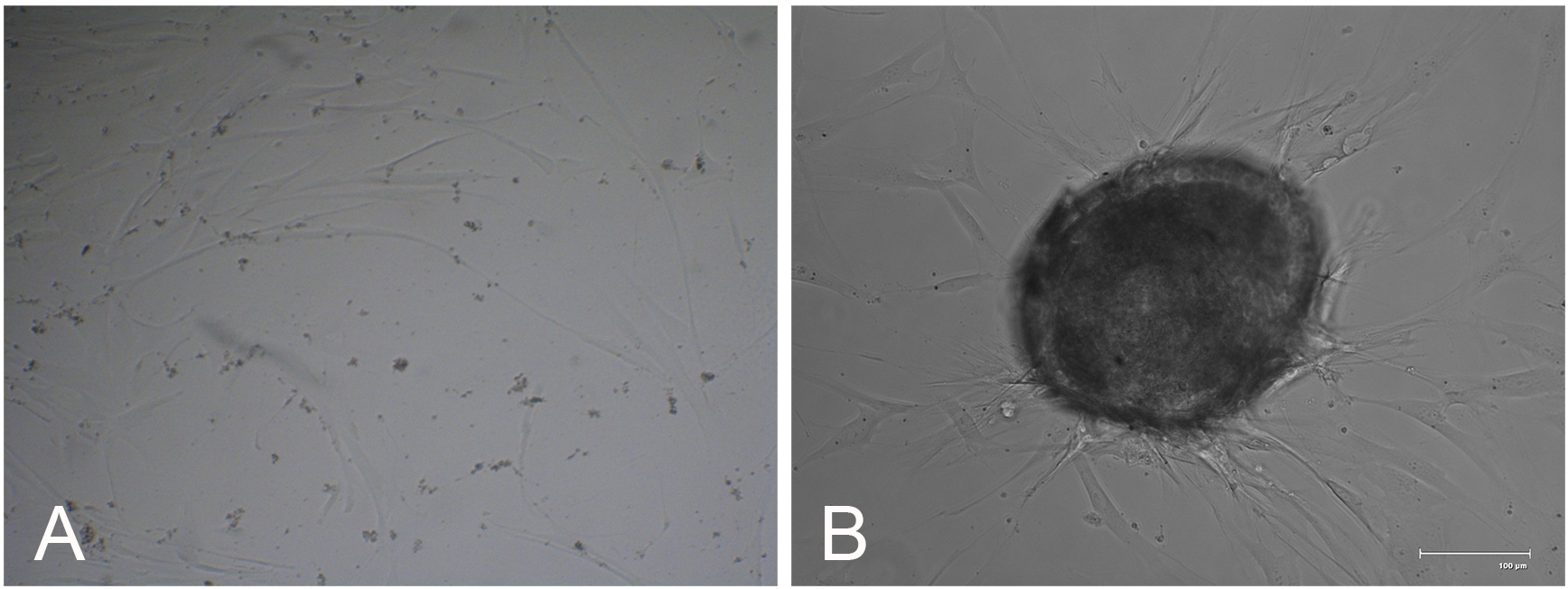
Figure 2. Sphere formation. Phase contrast microscopy showing isolated cells initially adherent to glass coverslips after seeding (A), which over the course of days to weeks aggregate and form sphere like structures (B). Scale bar = 100 µm.
- Using sterile technique in a sterile tissue culture hood, prepare 1 container of 70% ethanol and 1 container of PBS for tools and 1 container of PBS for washing. Place straight scissors, scalpel blade and forceps in 70% ethanol for 10 min.
- Preparation of tissue for sphere implantation
- Under sterile conditions within a biological safety cabinet (Heal Force Model HF-safe 1800) donor corneoscleral rims, which have been in storage at -80 °C for over 3 months, are divided into 1/8th segments, stored in individual 5 ml containers and re-frozen at -80 °C.
- Each corneoscleral rim is subjected to a total of 3 freeze-thaw cycles in order to decellularise the tissue prior to use for implantation.
Note: If complete decellularisation is required, LIVE/DEAD® 2 μM calcein AM and 4 μM ethidium homodimer-1 (Life Technologies) in standard culture medium can be used according to manufacturer directions to either confirm complete decellularisation or determine the need for additional freeze-thaw cycles.
- Under sterile conditions within a biological safety cabinet (Heal Force Model HF-safe 1800) donor corneoscleral rims, which have been in storage at -80 °C for over 3 months, are divided into 1/8th segments, stored in individual 5 ml containers and re-frozen at -80 °C.
- Corneal limbus microsurgery (Video 2)
Video 2. Surgical excision of a wedge of limbal tissue. Fine forceps were used with the non-dominant hand to anchor the sclera and provide counter-traction as two diagonal ~3 mm incisions were made prior to gripping the apex of the incised tissue to cut away and remove the anterior tissue segment. Cornea-limbus-sclera demarcations have to be visually approximated.- Using sterile technique in a laminar flow cabinet (Gelman model HLF 120 ), prepare 1 container of 70% ethanol and 1 container of sterile PBS for tools.
- Place fine forceps and MicroScalpel blade into the ethanol and leave for 10 min.
- Remove a thawed limbal rim segment and place into a Petri dish
- After washing tools in PBS, visualize the limbal rim using a binocular stereomicroscope.
- Using a MicroScalpel, make two diagonal ~3 mm incisions (Figure 3A), into the limbus with the apices intersecting such that a triangular wedge is created (Figures 3B and 3C). Using fine forceps grip the tissue at the apex and lift up the wedge (Figure 3D).
Figure 3. Surgical excision of a wedge of limbal tissue. Fine forceps were used with the non-dominant hand to anchor the sclera and provide counter-traction as two diagonal ~3 mm incisions (A) were being made (B, C) prior to gripping the apex to cut away the incised tissue (D). Cornea-limbus-sclera demarcations have to be visually approximated. - Cut away the anchoring tissue using the feather MicroScalpel while angling the blade anteriorly so that the depression created is shallower in the corneal direction (Figure 4).
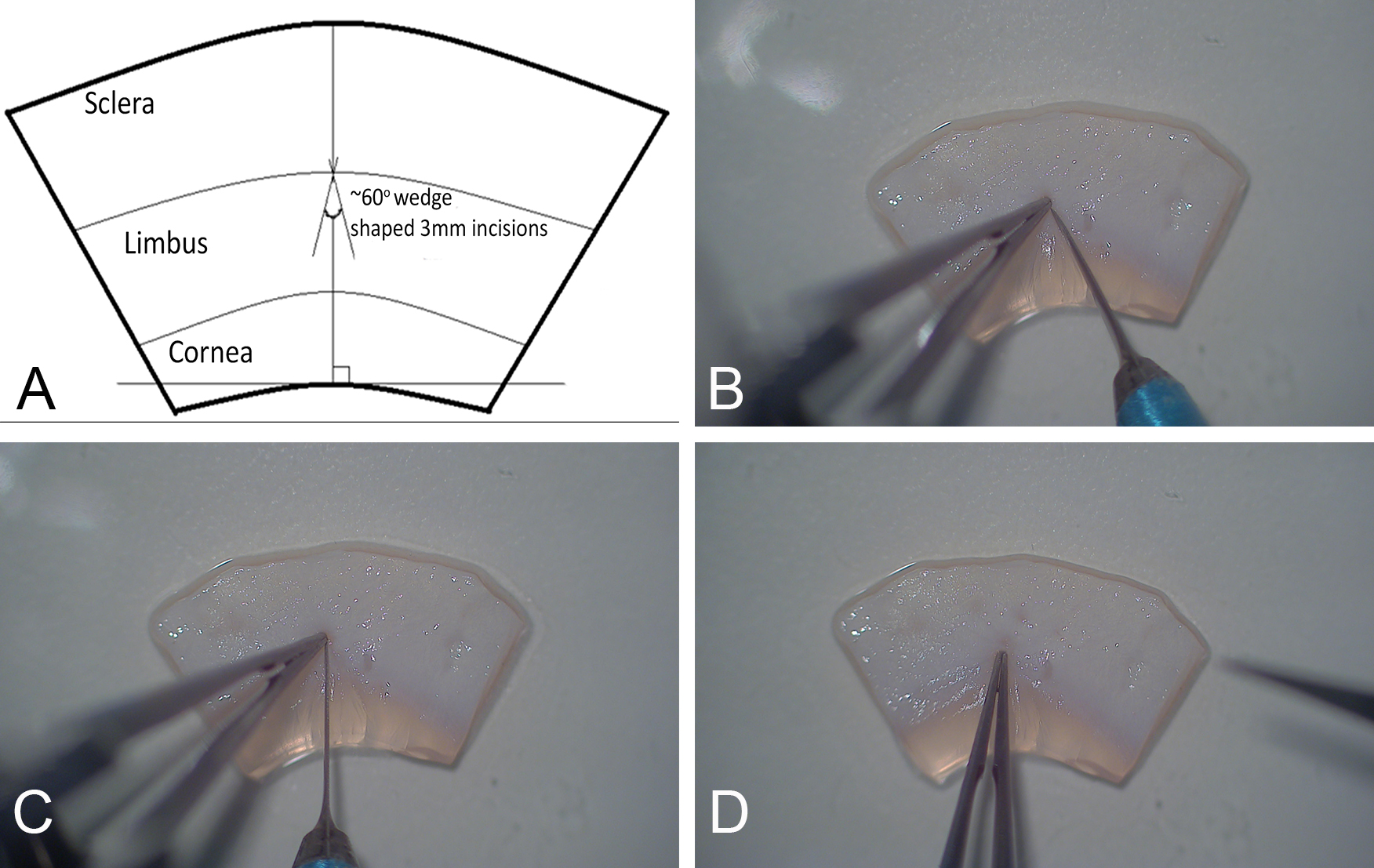
Figure 4. Removal of the wedge-shaped tissue. 3-D representation of the upward sloping floor of the wedge (A) that is created post excision (B) from the limbal region. - Repeat to create additional incisions in tissue.
Note: Visualization of the incisions are challenging initially to the untrained eye but will develop with practice. Good lighting will be helpful. - The Petri dish is closed and the tissue is transferred to a class II biological safety cabinet.
- Using sterile technique in a laminar flow cabinet (Gelman model HLF 120 ), prepare 1 container of 70% ethanol and 1 container of sterile PBS for tools.
- Sphere implantation
- Using sterile cotton buds, excess fluid is first blotted away from the corneoscleral tissue in which limbal wedge incisions have been made.
- Spheres are visualized in wells under a binocular stereomicroscope within a class II biological safety cabinet.
- Using a sterile 2-20 µl Eppendorf® Pipette set at a volume of 5 µl, gently aspirate spheres from culture.
- Visualize the incisions made in the corneoscleral tissue and eject the spheres and medium into the wedge incisions.
Note: It is best to eject as minimum possible volume of culture medium as possible while ensuring that spheres have been ejected. Occasionally, difficulties arise with spheres floating off the tissue if the sphere implantation, specifically the ejection of sphere from the micropipette, is not performed gently. - Transfer the corneoscleral rim with implanted sphere into a 35 x 10 mm cell culture dish and incubate at room temperature for 15 min epithelial side up.
- Invert the tissue so that the epithelium faces down.
Note: Through personal experience, inverting the tissue has proven to be better than culturing tissue the right side up as the cornea usually floats and the spheres become not fully submerged in culture medium which does not seem facilitate cell migration and survival. With inverted culture however, spheres occasionally do not remain in situ and become detached from the tissue. - Gently overlay the tissue with 2 ml of standard culture medium.
- Perform half medium changes every second day.
The following image panel and legend has been reproduced from Mathan et al. (2016) (Figure 5).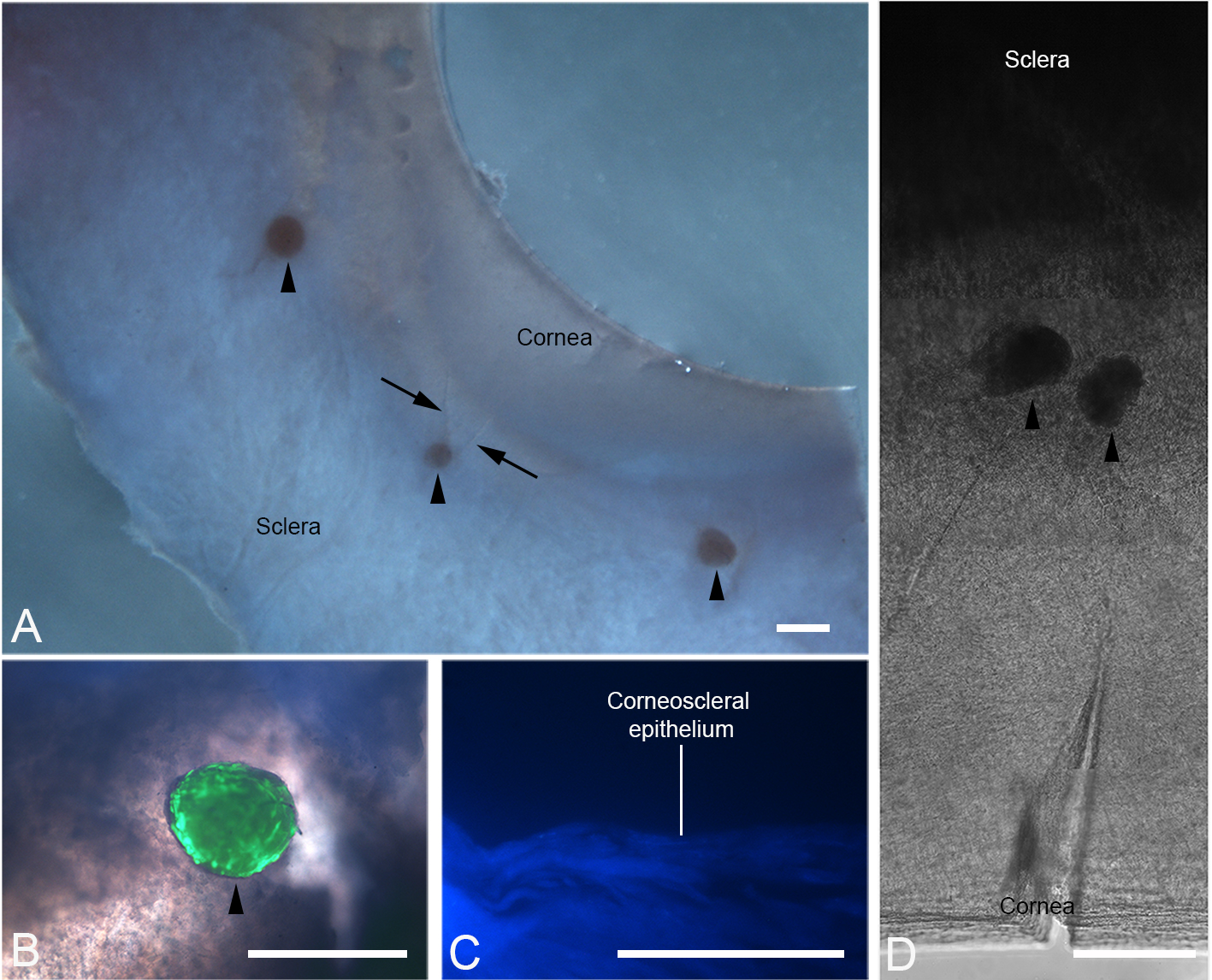
Figure 5. Implantation of peripheral corneal spheres into donor corneoscleral rims. Spheres (arrowheads) were implanted into wedge-shaped incisions made at the limbal region. Under stereomicroscopy, the corneoscleral rim with incisions (arrows) and implanted spheres can be clearly visualised (A). Combined phase contrast and fluorescence microscopy show an implanted sphere stained positively for live cells with LIVE/DEAD® stain (B). This signal is confined to the sphere and not detected in the surrounding tissue. A 40x DAPI stained 10 µm thick cross section of frozen-stored corneoscleral rim confirmed the absence of DAPI positive cell nuclei prior to implantation (C). Through phase contrast microscopy, the position of the spheres in the semi-transparent region of tissue is shown (D). Scale bars = 500 µm (A), 100 µm (B and D), 50 µm (C).
- Using sterile cotton buds, excess fluid is first blotted away from the corneoscleral tissue in which limbal wedge incisions have been made.
Data analysis
- To assess the viability of spheres and implanted cells in tissue, LIVE/DEAD® 2 μM calcein AM and 4 μM ethidium homodimer-1 (Life Technologies) in standard culture medium was used.
- Bright-field images, assessed using an SV6 Binocular Stereo microscope (Carl Zeiss), were captured using a NIKON Digital sight DS-UI camera (NIKON CORPORATION, Tokyo, Japan). Phase-contrast and fluorescence microscopy was performed using the following microscopes: Leica DMIL inverted contrasting microscope (Leica Microsystems, Wetzlar, Germany), 4x magnification 0.1 aperture, C PLAN with Leica Application Suite Version 4.4.0 Build 454; and Leica DM-RA upright fluorescence microscope (Leica Microsystems), 5x magnification 0.15 aperture, HC PL Fluotar and 40x magnification, 1.00 aperture, PL FLUOTAR Oil PH3 with NIS-Elements Br Microscope Imaging Software version 3.0 and images were captured using the NIKON Digital sight DS-UI camera (NIKON).
- The following image panel and legend has been reproduced from Mathan et al. (2016) (Figure 6).
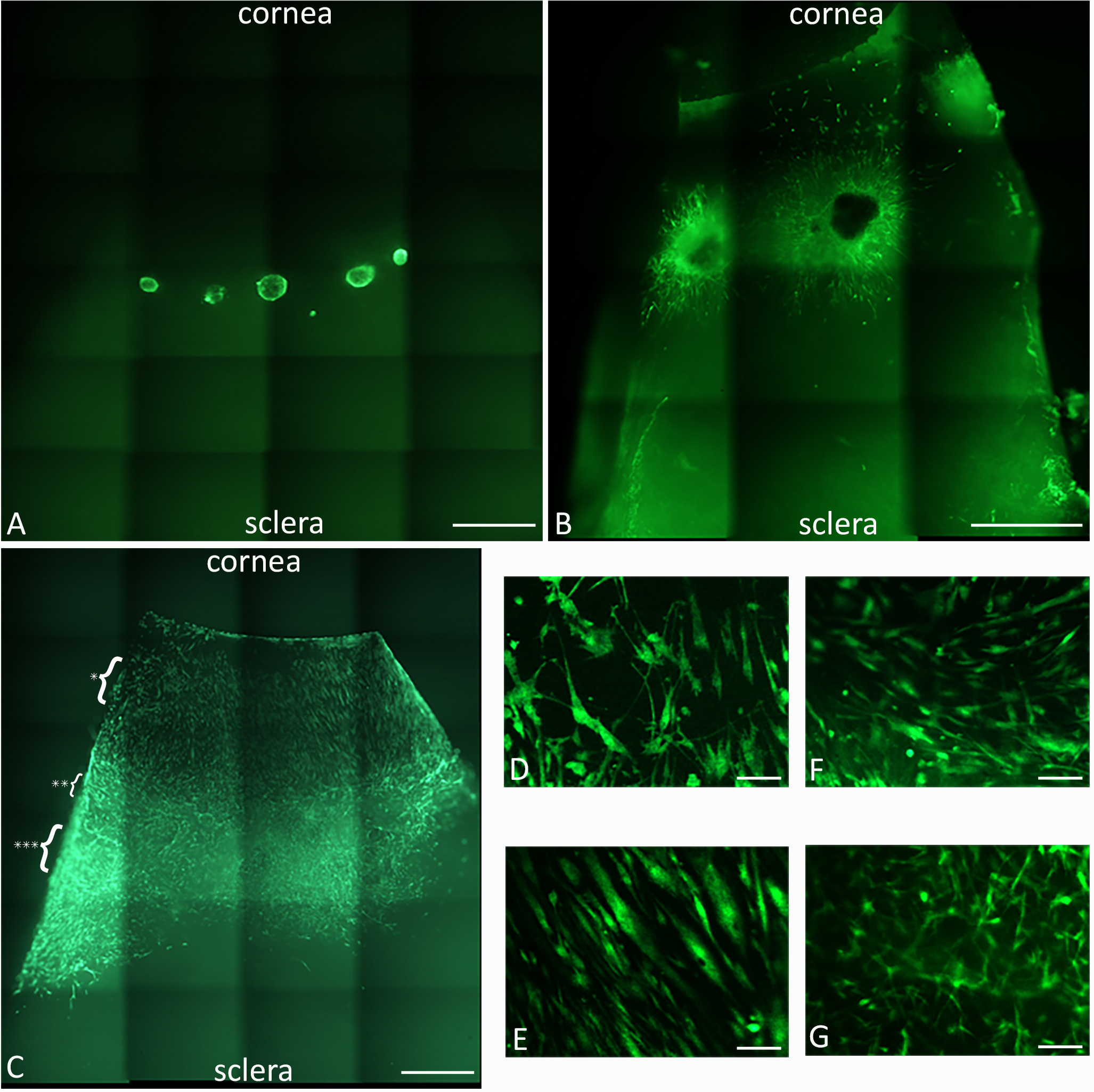
Figure 6. Peripheral corneal spheres implanted into corneoscleral tissue repopulate the ocular surface. LIVE/DEAD® staining (green) of implanted spheres, 5x magnification, at 0 h post implantation (A) and 4 days post implantation (B), show cell migration from the spheres appearing as green streaks out from the sphere. At 7 days post implantation (C), the entire corneal bed appears repopulated with live cells. Representative cells at the leading migratory edge of the corneal surface (D) and cells on the corneal surface taken from the region indicated by the single asterisk in panel C (E) show differing morphology. Representative cells over limbal region (F) and sclera (G) (taken from the region indicated by the double and triple asterisks respectively) show a different cell migration pattern and morphology to that observed in the corneal tissue. Scale bars = 1,000 µm (A, B, E). Scale bars = 100 µm (C, D, F, G).
Recipes
- Supplemented Neurobasal-A medium
Neurobasal-A medium supplemented with 2 ng/ml EGF, 1 ng/ml FGF, 1/50 B27, 1/100 N2, 2 µg/ml Heparin and 2 mM GlutaMAXTM - Standard culture medium
MEM (1x) GlutaMAX supplemented with 10% fetal calf serum and 1/100 Anti/Anti
Acknowledgments
The authors would like to thank the tissue donors and their families for their special gift to scientific research. They would also like to acknowledge Associate Professor Dipika Patel for assistance with the surgical method. The work presented in this study was funded by grants from the Auckland.
Medical Research Foundation [1111010], Save Sight Society [3622588], The University of Auckland School of Medicine PBRF grant as well as a Faculty of Medical and Health Sciences Summer Scholarship Award and John Hamel McGregor Award both to JM.
References
- Mathan, J. J., Ismail, S., McGhee, J. J., McGhee, C. N. and Sherwin, T. (2016). Sphere-forming cells from peripheral cornea demonstrate the ability to repopulate the ocular surface. Stem Cell Res Ther 7(1): 81.
- Yoon, J. J., Wang, E. F., Ismail, S., McGhee, J. J. and Sherwin, T. (2013). Sphere-forming cells from peripheral cornea demonstrate polarity and directed cell migration. Cell Biol Int 37(9), 949-960.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Mathan, J. J., Ismail, S., McGhee, J. J. and Sherwin, T. (2017). Implantation of Human Peripheral Corneal Spheres into Cadaveric Human Corneal Tissue. Bio-protocol 7(14): e2412. DOI: 10.21769/BioProtoc.2412.
Category
Stem Cell > Adult stem cell > Cell transplantation
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link