- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rice Lamina Joint Inclination Assay
(*contributed equally to this work) Published: Vol 7, Iss 14, Jul 20, 2017 DOI: 10.21769/BioProtoc.2409 Views: 13158
Reviewed by: Marisa RosaLaia ArmengotYuko Kurita

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Extraction and Quantification of Plant Hormones and RNA from Pea Axillary Buds
Da Cao [...] Christine A. Beveridge
Oct 5, 2022 2968 Views

Generating Reproducing Anoxia Conditions for Plant Phenotyping
Iny E. Mathew [...] Kendal D. Hirschi
Feb 5, 2023 1943 Views

ClearDepth Method for Evaluations of Root Depth in Soil-Filled Pots
Michel Ruiz Rosquete [...] Wolfgang Busch
Aug 20, 2025 2129 Views
Abstract
Brassinosteroids (BRs) promote rice lamina inclination. Recently, we showed that OsBUL1 knockout mutant rice (osbul1) is defective in brassinosteroid signaling (Jang et al., 2017). To show that lamina joint inclination of osbul1 is less-sensitive than WT to exogenous brassinolide (BL) treatment in the lamina joint inclination bioassays, we applied the protocol presented below. The protocol focuses on: (1) how to prepare rice samples for the assay, and (2) how to treat BL exogenously. Finally, we have added a result showing lamina inclination between WT and osbul1 in BL solutions of various concentrations.
Keywords: BioassayBackground
The rice lamina joint connects the leaf blade and sheath, contributing significantly to the leaf angle trait and BR is the main regulator of the trait, while other plant hormones, including ethylene, gibberellin, and auxin, also influence leaf angle (Gan et al., 2015). A more erect leaf facilitates the penetration of sunlight, enhancing photosynthetic efficiency and occupying less space in dense planting (Sakamoto et al., 2006). Thus, rice lamina inclination is one of the major agronomic traits affecting rice plant architecture. Actually, the rice lamina inclination assay developed mainly by Wada and his co-workers is a highly specific and sensitive bioassay for BRs (Wada et al., 1981 and 1984). In this bioassay, treatment with BRs induces greater cell expansion of adaxial cells relative to the abaxial cells in the joint regions, causing laminar inclination in a concentration-dependent manner (Takeno and Pharis, 1982; Cao and Chen, 1995). Changes in cell wall extensibility or loosening are essential for cell expansion (Campbell and Braam, 1999). Although the molecular mechanism for such action remains elusive, cell wall loosening enzymes including xyloglucan endotransglycosylase have been shown to be upregulated by BL and involved in this modification, resulting in laminar inclination in rice (Uozu et al., 2000). Thus, here we describe a procedure through which we could distinguish the BR sensitivity between the wild type and erect leafed osbul1 mutant plants through the rice lamina inclination assay.
Materials and Reagents
- 250 µl pipette tips (Mettler-Toledo International, Rainin, catalog number: 17007479 )
- 1 ml pipette tips (Mettler-Toledo International, Rainin, catalog number: 17001121 )
- 50 ml SuperClear centrifuge tube (Labcon, catalog number: LAB3181 )
- Filter paper (Advantec, No.1: 90 mm)
- Petri dish, round, 90 x 15 mm (Alpha Plus, catalog number: 16001-1 )
- 50 ml syringe (Sigma-Aldrich, catalog number: Z124990 )
- Syringe filter (VWR, catalog number: 89041-306 )
- Micropore tape (3M, catalog number: 1530-0 )
- 1 ml tubes
- Rice seeds: Orysa sativa spp. japonica cv. Hwayoung and OsBUL1 knockout mutant rice (osbul1)
- Ethanol (Avantor Performance Materials, J.T. Baker®, catalog number: 8006 )
- Sodium hypochlorite (NaOCl, Commercial Bleach–CLOROX)
- Tween 20 (Alfa Aesar, Affymetrix/USB, catalog number: J20605 )
- Potassium hydroxide (KOH) (SHOWA, catalog number: 1637-0150 )
- Murashige & Skoog basal medium with Vitamins (MS) (PhytoTechnology Laboratories, catalog number: M519 )
- Sucrose (Alfa Aesar, Affymetrix/USB, catalog number: J21938 )
- Phytogel (Sigma-Aldrich, catalog number: P8169-500G )
- Brassinolide (BL) (Sigma-Aldrich, catalog number: E1641 )
- Sodium hypochlorite solution (with final available chlorine of 2%) (see Recipes)
- 5 N potassium hydroxide (KOH) solution (see Recipes)
- Murashige & Skoog (MS) media (see Recipes)
- 1 mM Brassinolide (BL) stock solution (see Recipes)
Equipment
- Rice husker (KETT ELECTRIC LABORATORY, model: TR-130 )
- Ultrasonic cleaner (Elma, model: E-30H )
- Clean bench (Chu-An, model: MBH-420N )
- Scissors (Basic Life, catalog number: 76000 )
- Forceps (Basic Life, catalog number: BL6502 )
- Growth chamber (CHANG KUANG, model: CK-68EX )
- Digital camera (Sony, model: NEX-3N )
- Protractor (Taiwan united stationery, catalog nunber: HA401 )
- 600 ml beaker (DWK Life Sciences, DURAN, catalog number: 21 106 48 )
- Glass petri dish (Sun Chion, catalog number: B16A1-0090 )
- Autoclave
- 10 ml measuring cylinders (DWK Life Sciences, DURAN, catalog number: 21 390 08 04 )
- 100 ml measuring cylinders (DWK Life Sciences, DURAN, catalog number: 21 390 24 02 )
- 500 ml measuring cylinders (DWK Life Sciences, DURAN, catalog number: 21 390 44 03 )
- Vortex mixer (Vortex-Genie2, Scientific Industries, model: Model G560 )
- Incubator (YIHDER TECHNOLOGY, model: LM-570RD )
- Pipetmans (Gilson, models: P20 , P200 and P1000 )
- RiOsTM Essential 16 Water Purification System (EMD Millipore, model: RiOsTM Essential 16 )
- Summit Series Analytical Balance (Denver Instrument, model: SI-234 )
- pH meter (UltraBasic Benchtop pH Meter, Denver Instrument, model: UB-10 )
Software
- ImageJ (https://imagej.nih.gov/ij/) for lamina angle measurement
Procedure
- Seedling preparation for lamina inclination
- Surface sterilize rice seeds
- Remove the lemma and palea of seeds using a rice husker (Figure 1).

Figure 1. Rice husker used - Put 50-60 naked seeds into a 50 ml SuperClear centrifuge tube and sterilize the surface of the seeds with 30 ml of 70% ethanol for 1 min and vigorously shake by hand.
- Rinse the seeds with 30 ml sterile water and pour off the dirty liquid.
- Add 30 ml of 2% sodium hypochlorite solution (see Recipes) and place the tube in an ultrasonic cleaner for 20 min.
- Discard the sodium hypochlorite solution and wash the seeds with 30 ml sterile water in the clean bench. We usually repeat the washing process 10-15 times.
- Place the seeds on autoclaved filter paper to dry and then transfer 20 seeds into a beaker containing MS media (see Recipes) using sterile forceps in the clean bench.
- Remove the lemma and palea of seeds using a rice husker (Figure 1).
- Germinate the sterilized seeds inside a growth chamber under long days (16 h light) at 28 °C for 8 days.
- Sample uniform seedlings (based on similar height in each genotype) by excising approximately 2 cm segments (Figure 2) containing the second-leaf lamina joint, leaf blade and leaf sheath and float excised samples on sterile water for 10 min before transferring them to BL solution.
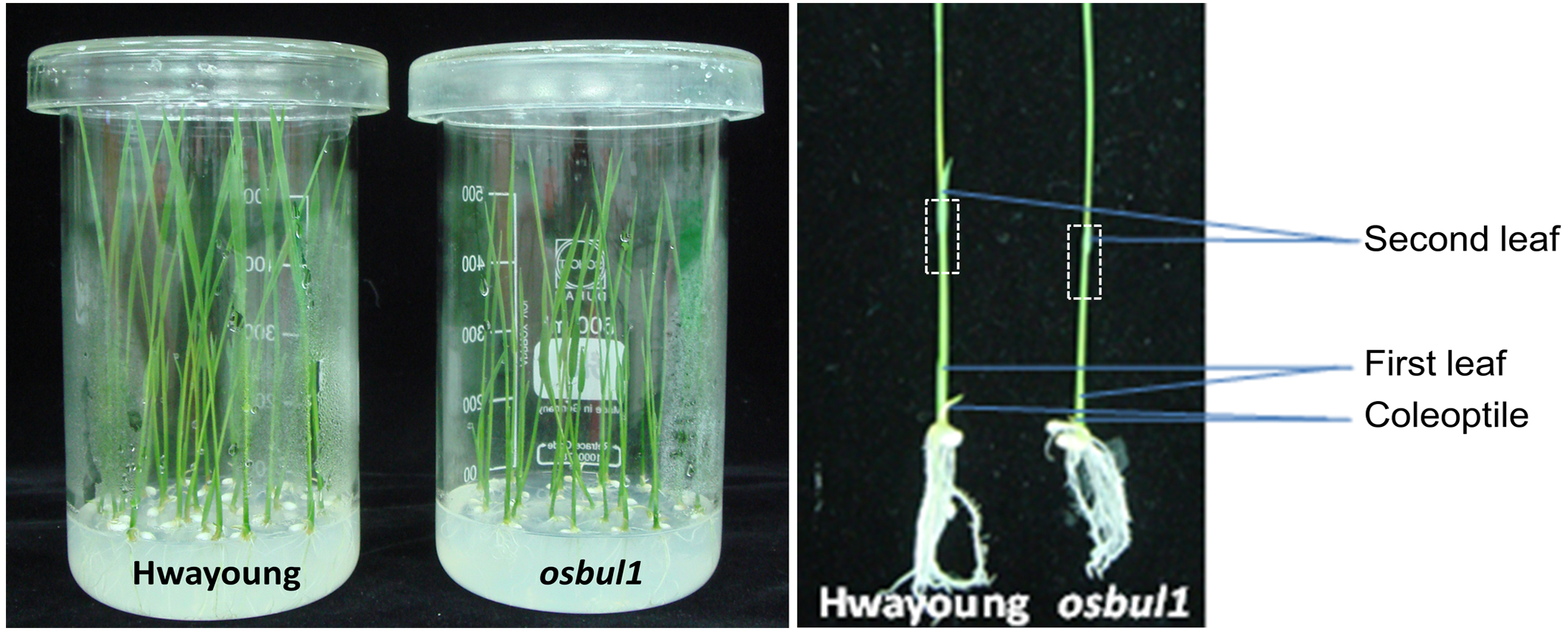
Figure 2. Eight-day-old rice seedlings for lamina inclination assay. WT (Hwayoung cultivar) and osbul1 mutant rice were grown in beakers containing MS media (left). Leaf segments containing the second-leaf lamina joint of rice seedlings are marked by boxes outlined with dotted lines, which were used for lamina inclination assay.
- Surface sterilize rice seeds
- Exogenous BL treatment
- Put 20 ml of each test BL solution at designated concentrations (0 M, 10-6 M, 10-7 M, 10-8 M and 10-9 M in water) in 90 x 15 mm Petri dishes. The solution should be prepared immediately before use.
- Float leaf lamina segments on BL solution (see Recipes) (0 M, 10-6 M, 10-7 M, 10-8 M and 10-9 M) in an incubator at 29 °C in the dark for 2 days (Figure 3).
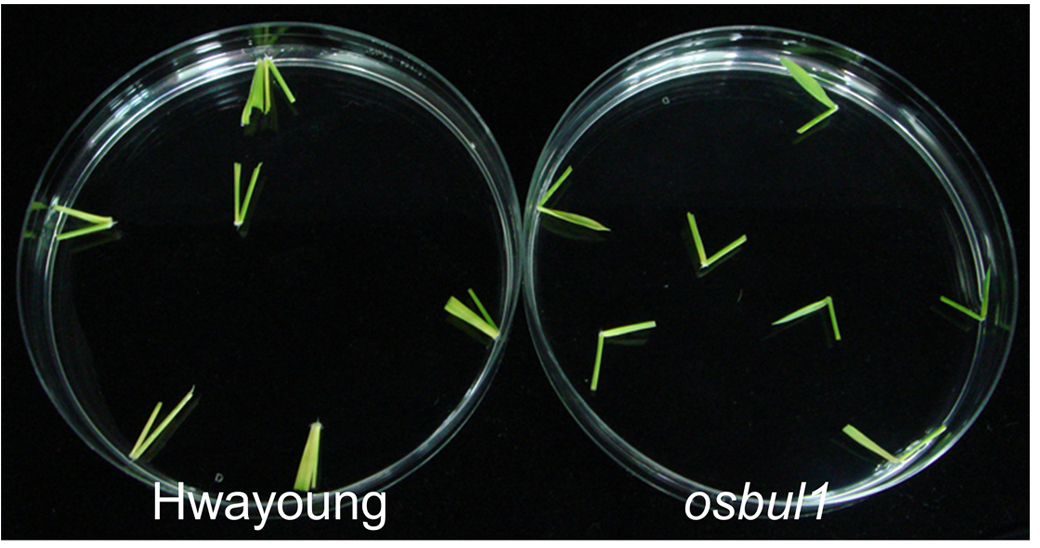
Figure 3. BR-induced lamina joint inclination in WT (Hwayoung cultivar) and OsBUL1 knock-out mutant (osbul1) rice. The photo was taken after BL (10-6 M) treatment for 2 days. - After 2 days, take sample photos with a digital camera, print them and measure the angle induced between the lamina and the sheath (degree of angle of leaf blade against the axis of leaf sheath) with a protractor (Figure 4).
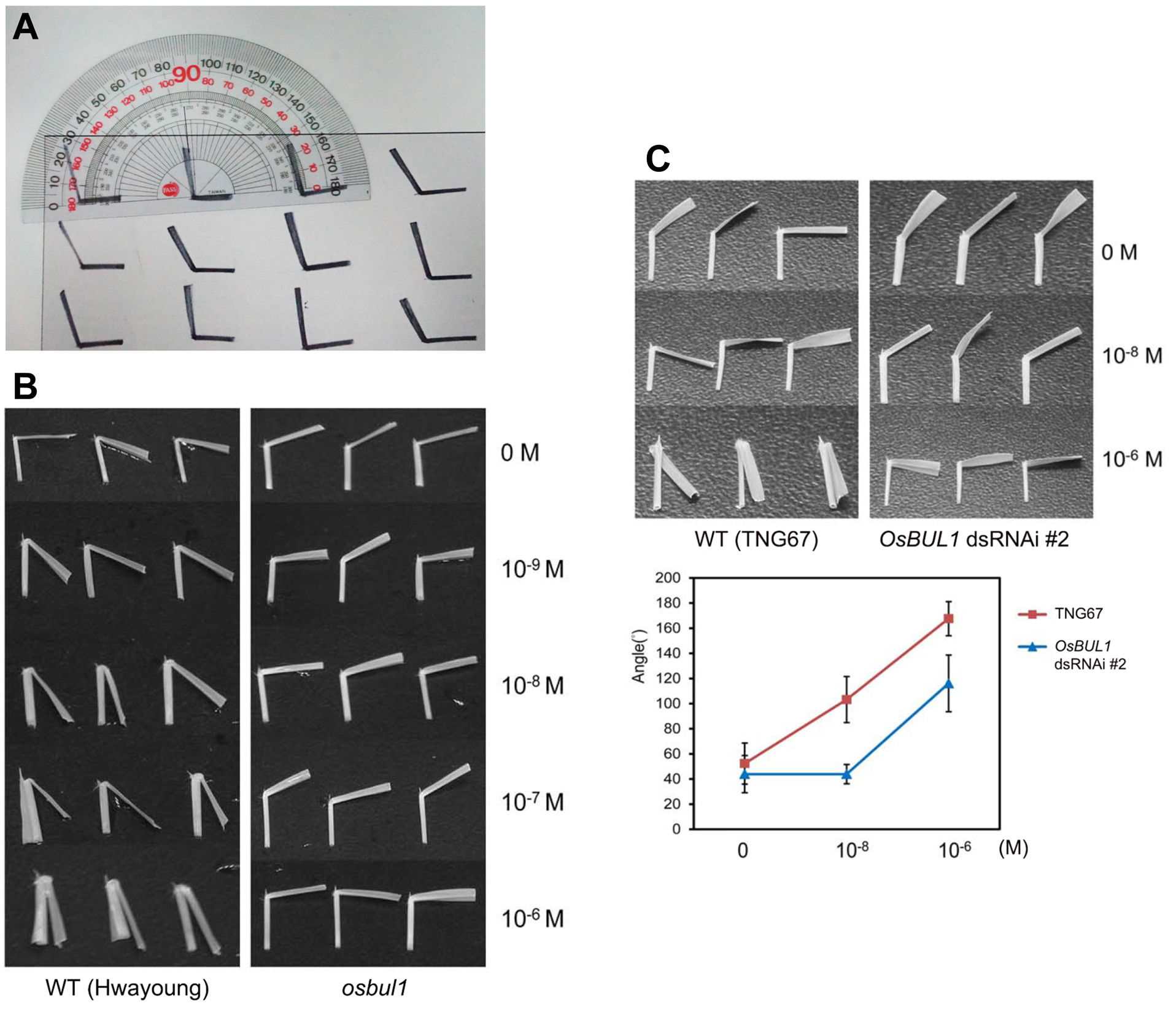
Figure 4. Measurement of lamina angles. A. Measuring the lamina angle with a protractor; B. Representative leaf segments containing lamina joint, blade and sheath from WT (Hwayoung cultivar) and OsBUL1 KO (osbul1) plants after incubation for 48 h in the BL solution at different concentrations. C. Reduced leaf angles of OsBUL1 dsRNAi line (#2) compared to WT (TNG67 cultivar) in 0 M, 10-8 M and 10-6 M BL solution (*P < 0.001).
- Put 20 ml of each test BL solution at designated concentrations (0 M, 10-6 M, 10-7 M, 10-8 M and 10-9 M in water) in 90 x 15 mm Petri dishes. The solution should be prepared immediately before use.
Data analysis
The same number of rice seedlings was used for each BL concentration set (n = 6-12). When ImageJ software was used for lamina angle measurement, photo files containing rice lamina fragments were opened in the program. By using the angle tool in the software (https://imagej.nih.gov/ij/docs/tools.html), 3 points, one each for the lamina blade, joint and sheath in the lamina fragment were fixed and the angle made by the three points was measured (Figure 5). Measured angle values are presented as means with standard deviations using Microsoft Office 2011 Excel (Figure 4C). The Student’s t-test is used for statistical significance. Data from at least three independent repeats were obtained.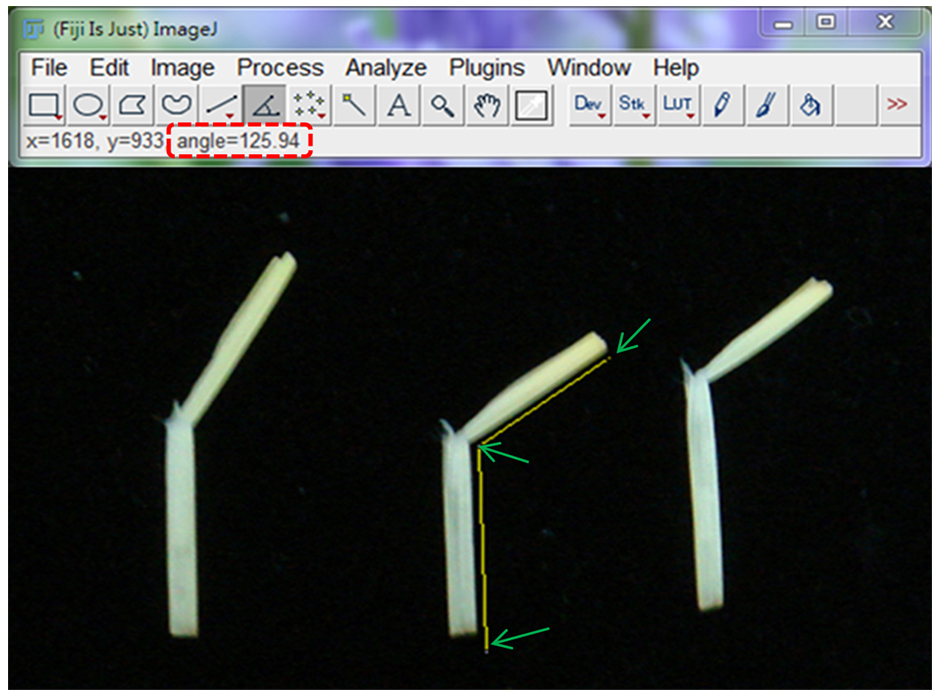
Figure 5. Measurement of lamina angles using the ImageJ program. The three points are marked by green arrows using the angle tool. The measured angle is marked by the box with a dashed red border.
Notes
BL solution should be freshly prepared. Since the sensitivity of this test varied within the rice cultivars employed, crossed data analyses with different rice cultivars should be avoided. For lamina angle measurement, image processing computer programs such as ImageJ can also be used.
Recipes
- Sodium hypochlorite solution (with final available chlorine of 2%) for 30 ml
Add 10 ml of commercial Bleach (CLOROX) into 20 ml of sterile water containing a few drops of Tween 20 - 5 N potassium hydroxide (KOH) solution for 30 ml
Add 8.417g KOH into 30 ml distilled water and filter the solution with a syringe filter - Murashige & Skoog (MS) media for 500 ml
2.2 g Murashige & Skoog basal medium with Vitamins
15 g sucrose
Distilled water up to 500 ml
Adjust pH to 5.7 with a few drops of 5 N KOH, and distribute 100 ml into a 600 ml beaker containing 0.3 g phytogel each, and then cover the beaker with a glass Petri dish. Seal the beaker with 3M micropore tape and autoclave - 1 mM Brassinolide (BL) stock solution
- Dissolve 2 mg of BL (molecular weight 480.68) in 1 ml of ethanol
- Add 3.16 ml of distilled water to get 1 mM final concentration
- Then split into aliquots in 1 ml tubes and store at -20 °C
- Serial dilution: e.g., to make 10-4 M BL solution, 1 ml of BL (10-3 M) mixed with 9 ml of sterile water becomes 10 ml of 10-4 M BL solution
Acknowledgments
This protocol was adapted from the method for rice lamina inclination assay described by Jang et al. (2017). We thank Ms. Miranda Loney for help with English editing. This work was supported in part by a core grant from BCST of ABRC, Academia Sinica.
References
- Campbell, P. and Braam, J. (1999). Xyloglucan endotransglycosylases: diversity of genes, enzymes and potential wall-modifying functions. Trends Plant Sci 4(9): 361-366.
- Cao, H. and Chen, S. (1995). Brassinosteroid-induced rice lamina joint inclination and its relation to indole-3-acetic acid and ethylene. Plant Growth Regul 16(2): 189-196.
- Gan, L., Wu, H., Wu, D., Zhang, Z., Guo, Z., Yang, N., Xia, K., Zhou, X., Oh, K., Matsuoka, M., Ng, D. and Zhu, C. (2015). Methyl jasmonate inhibits lamina joint inclination by repressing brassinosteroid biosynthesis and signaling in rice. Plant Sci 241: 238-245.
- Jang, S., An, G. and Li, H. Y. (2017). Rice leaf angle and grain size are affected by the OsBUL1 transcriptional activator complex. Plant Physiol 173(1): 688-702.
- Sakamoto, T., Morinaka, Y., Ohnishi, T., Sunohara, H., Fujioka, S., Ueguchi-Tanaka, M., Mizutani, M., Sakata, K., Takatsuto, S., Yoshida, S., Tanaka, H., Kitano, H. and Matsuoka, M. (2006). Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24(1): 105-109.
- Takeno, K. and Pharis, R. P. (1982). Brassinosteroid-induced bending of leaf lamina of dwarf rice seedlings: an auxin-mediated phenomenon. Plant Cell Physiol 23(7): 1275-1281.
- Uozu, S., Tanaka-Ueguchi, M., Kitano, H., Hattori, K. and Matsuoka, M. (2000). Characterization of XET-related genes of rice. Plant Physiol 122(3): 853-859.
- Wada, K., Marumo, S, Abe, H., Morishita, T., Nakamura, K., Uchiyama, M. and Mori, K. (1984). A rice lamina inclination test–a micro-quantitative bioassay for brassinosteroids. Agric Biol Chem 48(3): 719-726.
- Wada, K., Marumo, S., Ikekawa, N., Morisaki, M. and Mori, K. (1981). Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant Cell Physiol 22(2): 323-325.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, H., Wang, H. and Jang, S. (2017). Rice Lamina Joint Inclination Assay. Bio-protocol 7(14): e2409. DOI: 10.21769/BioProtoc.2409.
- Jang, S., An, G. and Li, H. Y. (2017). Rice Leaf Angle and Grain Size Are Affected by the OsBUL1 Transcriptional Activator Complex. Plant Physiol 173(1): 688-702.
Category
Plant Science > Plant physiology > Phenotyping
Plant Science > Plant biochemistry > Plant hormone
Biochemistry > Other compound > Plant hormone > Brassinosteroid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










