- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Non-radioactive LATS in vitro Kinase Assay
Published: Vol 7, Iss 14, Jul 20, 2017 DOI: 10.21769/BioProtoc.2391 Views: 12448
Reviewed by: Ralph BottcherHsin-Yi ChangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Purification of Native Acetyl CoA Carboxylase From Mammalian Cells
Yaxue Sun [...] Hongtao Zhu
Feb 20, 2025 2193 Views

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2282 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 496 Views
Abstract
This protocol describes a method to directly measure LATS activity by an in vitro kinase assay using YAP as a substrate.
Keywords: LATSBackground
Large tumor suppressor 1/2 (LATS1/2) are protein kinases and core components of the Hippo pathway, which regulates organ size and tissue homeostasis. LATS kinase is activated by phosphorylation on its hydrophobic motif (HM, Thr 1079 for LATS1 and Thr 1041 for LATS2). As a result, Western blotting with phosphoantibody recognizing LATS at HM provides an indirect way to assess LATS kinase activity (Data analysis, Figure 1). In addition, active LATS phosphorylates and inhibits the transcription co-activator Yes-associated protein (YAP) at Ser 127, leading to YAP binding to 14-3-3 and cytoplasmic retention (Zhao et al., 2007). Using YAP as a substrate in LATS in vitro kinase assay provides a method to directly assess LATS kinase activity. Through this assay, we were able to show that serum starvation and sorbitol-induced osmotic activate LATS (Yu et al., 2012; Hong et al., 2017) and further lead to YAP Ser 127 phosphorylation (Data analysis, Figure 2).
Materials and Reagents
- Pipette tips
- 10 cm plates
- 1.5 ml Eppendorf tube
- Dialyzer (EMD Millipore, catalog number: 71507-3 )
- pGEX-KG-GST-YAP plasmid (Addgene, catalog number: 33052 )
- BL21(DE3) competent cells (Agilent Technologies, catalog number: 230134 )
- HEK293A cells
- LB broth (Fisher Scientific, catalog number: BP1426-2 )
- Carbenicillin disodium salt (Sigma-Aldrich, catalog number: 205805-250MG )
- Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: I5502 )
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010049 )
- Protease inhibitor cocktail tablet (Roche Diagnostics, catalog number: 11873580001 )
- Phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626-5G )
- Dithiothreitol (DTT) (Bio-Rad Laboratories, catalog number: 1610611 )
- Triton X-100 (Sigma-Aldrich, catalog number: T9284 )
- Glutathione Sepharose 4B (GE Healthcare, catalog number: 17075601 )
- Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11965092 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- Phosphatase inhibitor mini tablet (Thermo Fisher Scientific, Thermo ScientificTM , catalog number: 88667 )
- Protein A/G magnetic beads (Thermo Fisher Scientific, Thermo ScientificTM , catalog number: 88802 )
- Kinase buffer (New England Biolabs, catalog number: B6022S )
- Cold ATP (Sigma-Aldrich, catalog number: A2383 )
- Sorbitol (Fisher Scientific, catalog number: S459-500 )
- Antibodies
- Lats1 antibody (Cell Signaling Technology, catalog number: 3477S )
- pYAP S127 antibody (Cell Signaling Technology, catalog number: 4911S )
- pLATS-HM antibody (Cell Signaling Technology, catalog number: 8654S )
- GAPDH antibody (Santa Cruz Biotechnology, catalog number: sc-25778 )
- GST antibody (Sigma-Aldrich, catalog number: SAB4200237 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3294 )
- Tris-base (Fisher Scientific, catalog number: BP152-10 )
- L-glutathione reduced (Sigma-Aldrich, catalog number: G4251 )
- β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-10 )
- Glycerol (Fisher Scientific, catalog number: G33-1 )
- Sodium lauryl sulfate (SDS) (Fisher Scientific, catalog number: S529-500 )
- Bromophenol blue (Bio-Rad Laboratories, catalog number: 1610404 )
- Sodium fluoride (NaF) (Acros Organics, catalog number: 424325000 )
- EDTA (Mediatech, catalog number: 46-034-CI )
- NP-40 substitute (Sigma-Aldrich, catalog number: 74385 )
- Elution buffer (see Recipes)
- Dialysis buffer (see Recipes)
- 4x SDS sample buffer (see Recipes)
- Mild lysis buffer (see Recipes)
- TBS buffer (see Recipes)
Equipment
- Pipettes
- Spectrophotometer (Biochrom, model: Biochrom WPA Biowave II )
- Centrifuge (Thermo Fisher Scientific, Thermo ScientificTM , model: SorvallTM RC 6 Plus)
- Sonic dismembrator (Sonicator) (Fisher Scientific, model: Model 505 )
- Bench-top centrifuge (Denville Scientific, model: Denville 300D , catalog number: C0265-24)
- Magnetic rack (Thermo Fisher Scientific, catalog number: 12321D )
- Shaker (Eppendorf, New BrunswickTM, model: Excella® E24 )
- Heat block
Procedure
- Purification of GST-YAP protein
- Transform pGEX-KG-GST-YAP in BL21(DE3) competent cells by heat shock at 42 °C for 45 sec.
- Plate the cells on LB agar plates with 100 μg/ml carbenicillin and incubate overnight at 37 °C.
- Pick a single colony and inoculate in 5 ml LB broth with carbenicillin. Incubate overnight at 37 °C.
- Pour the 5 ml cultured bacteria in 500 ml LB broth and grow at 37 °C for around 2-3 h until OD600 = 0.6-0.8.
- Induce with 0.1-0.4 mM IPTG. Shake overnight at 16 °C.
- Spin down the bacteria at 4,000 x g for 15 min at 4 °C.
- Lyse the cells in 9 ml of PBS + 1x protease inhibitor + 1 mM PMSF + 1 mM DTT.
- Sonicate on ice. Total sonication time 1 min 30 sec, interval 15 sec. Amp = 30%.
- Add 1% Triton-X to the sonicated bacterial samples. Shake in 4 °C for 20-30 min.
- At the meantime, wash the glutathione Sepharose 4B beads 3 times with 0.1% Triton-X in PBS. 200 μl beads were used for 500 ml of bacteria culture.
- Spin down the bacteria at 10,000 x g for 30 min at 4 °C.
- Collect supernatant and add the beads. Rotate overnight at 4 °C.
- Centrifuge down the beads at 500 x g for 5 min at 4 °C.
- Wash with PBS + 0.1% Triton-X 3 times, 5 min each.
- Add 500 μl elution buffer and rotate for 1 h at 4 °C.
- Spin down the sample at 500 x g for 5 min at 4 °C.
- Repeat elution again.
- Collect the elution for dialysis against dialysis buffer at 4 °C twice.
- Snap freeze the protein and store at -80 °C
- Transform pGEX-KG-GST-YAP in BL21(DE3) competent cells by heat shock at 42 °C for 45 sec.
- LATS in vitro kinase assay
- HEK293A cells are cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS).
- Cells were seeded in 10 cm plates at a density of 1.5 x 106 per plate and were incubated in 37 °C, 5% CO2 until 80% confluent. A positive control can be cells that are serum starved for 30 min (Figure 2).
- Wash the cells with cold PBS.
- Remove PBS and scrape down the cells with 1 ml cold Mild lysis buffer (see Recipes) supplemented with 1x protease inhibitor, 1x phosphatase inhibitor, and 1 mM PMSF. Transfer the cells into 1.5 ml Eppendorf tubes.
- Incubate the samples on ice for 10 min.
- Spin down the lysates at 12,000 x g for 15 min at 4 °C.
- Collect the supernatants and incubate with 1 μl LATS1 antibody per sample overnight at 4 °C with constant rotation.
- Using a magnetic rack, wash the magnetic beads (10 μl per sample) 3 times with Mild lysis buffer. Resuspend the magnetic beads in 50 μl of Mild lysis buffer and add to the lysate. Rotate for 1 h at 4 °C.
- Using a magnetic rack, wash samples 3 times (10 min each) with 1 ml Mild lysis buffer per sample at 4 °C.
- Wash samples once with 1 ml 1x TBS (see Recipes) per sample for 10 min at 4 °C.
- While washing with TBS, prepare a master mix for the kinase reaction. Each kinase reaction contains 4 μl 10x kinase buffer, 500 μM cold ATP, and 1 μg GST-YAP in 40 μl reaction mix.
- Spin down the samples and remove the TBS using a magnetic rack. Add 40 μl of master mix into each reaction.
- Shake the reaction mixture at 30 °C for 30 min.
- Add 4x SDS sample buffer to stop the reaction, and heat the samples at 100 °C for 5 min.
- The samples are then subjected to SDS-PAGE and Western blotting.
- HEK293A cells are cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS).
Data analysis
Sorbitol or serum starvation-induced LATS activity can be assayed by detecting the phosphorylation of LATS at HM (Figure 1). Direct kinase activity of LATS can be measured by detecting the phosphorylation level of YAP in LATS in vitro kinase assay (Figure 2). Increased YAP phosphorylation represents increased LATS kinase activity.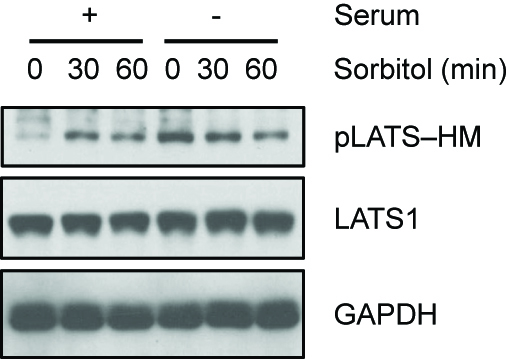
Figure 1. Serum starvation and sorbitol-induced osmotic stress induce LATS HM phosphorylation. HEK293A cells were treated with 0.2 M sorbitol for 30 or 60 min in the presence or absence of serum. LATS phosphorylation at the hydrophobic motif (HM) was detected with the phospho-specific pLATS antibody. (pLATS-HM Ab 1:1,000 dilution, LATS1 Ab 1:2,000 dilution, GAPDH Ab 1:2,000 dilution in 5% BSA containing TBST).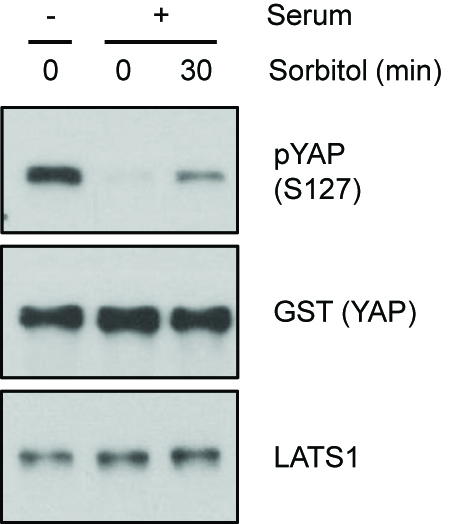
Figure 2. Kinase activity measured by LATS in vitro kinase assay. HEK293A cells were serum starved or treated with 0.2 M sorbitol for 30 min. LATS1 was immunoprecipitated and an in vitro kinase assay was performed using recombinant GST-YAP as a substrate. Phosphorylation of YAP was determined by immunoblotting with a phospho-YAP (S127) antibody. (pYAP S127 Ab 1:1,000 dilution, GST Ab 1:2,000 dilution, LATS1 Ab 1:2,000 dilution in 5% BSA containing TBST).
Recipes
- Elution buffer
50 mM Tris pH 8.0
20 mM glutathione
Note: Prepared fresh. - Dialysis buffer
20 mM Tris pH 8.0
2 mM β-mercaptoethanol
100 mM NaCl
10% glycerol
Note: Prepared fresh. - 4x SDS sample buffer
200 mM Tris pH 6.8
8% SDS
0.1% bromophenol blue
40% glycerol
20% β-mercaptoethanol
Note: Stored at room temperature. - Mild lysis buffer
20 mM Tris pH 7.5
100 mM NaCl
50 mM NaF
2 mM EDTA
1% NP-40 substitute
Note: Stored at 4 °C. - TBS buffer
20 mM Tris-HCl pH 7.5
170 mM NaCl
Note: Stored at room temperature.
Acknowledgments
This protocol was adapted from and used in Yu et al. (2012) and Hong et al. (2017). A.W.H. is supported in part by the T32 GM007752 training grant. K.L.G. is supported by grants from National Institute of Health (CA196878, GM051586)
References
- Hong, A. W., Meng, Z., Yuan, H. X., Plouffe, S. W., Moon, S., Kim, W., Jho, E. H. and Guan, K. L. (2017). Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep 18(1): 72-86.
- Yu, F. X., Zhao, B., Panupinthu, N., Jewell, J. L., Lian, I., Wang, L. H., Zhao, J., Yuan, H., Tumaneng, K., Li, H., Fu, X. D., Mills, G. B. and Guan, K. L. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150(4): 780-791.
- Zhao, B., Wei, X., Li, W., Udan, R. S., Yang, Q., Kim, J., Xie, J., Ikenoue, T., Yu, J., Li, L., Zheng, P., Ye, K., Chinnaiyan, A., Halder, G., Lai, Z. C. and Guan, K. L. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21(21): 2747-2761.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hong, A. W. and Guan, K. (2017). Non-radioactive LATS in vitro Kinase Assay. Bio-protocol 7(14): e2391. DOI: 10.21769/BioProtoc.2391.
Category
Cancer Biology > Cancer biochemistry > Protein
Biochemistry > Protein > Activity
Cell Biology > Cell signaling > Phosphorylation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









