- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transplantation of Embryonic Cortical Tissue into Lesioned Adult Brain in Mice
Published: Vol 7, Iss 12, Jun 20, 2017 DOI: 10.21769/BioProtoc.2360 Views: 8699
Reviewed by: Xi FengEdel HennessyJingang Huang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1645 Views

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2677 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1660 Views
Abstract
Transplantation of embryonic cortical tissue for repairing the damaged brain has provided a potential therapy for brain injury and diseases. The grafted tissue can successfully survive and participate in reestablishing the functional neural circuit of the host brain. Transplantation surgery can be combined with fluorescently labeled transgenic mice to evaluate the reconstruction of neuronal network (Falkner et al., 2016) and the repopulation of a subset of cortical cells. By using this approach, we have shown that infiltrating cells from host brain can restore the microglial population in the graft tissue (Wang et al., 2016). This protocol describes the detailed procedure of the transplantation surgery in mice, including establishing a lesion model in the host brain, preparing the embryonic cortical graft, and transplanting the embryonic cortical graft to adult brain.
Keywords: TransplantationBackground
Most neurons in adult brain are post mitotic cells and are not capable of regenerating new daughter cells, this results in a limited ability of self-repairing of adult brain after suffering from brain injury or diseases. Replacing the damaged brain tissue with embryonic neural graft is one of the potential effective therapies to repair the damaged neural pathways in the adult brain (Tuszynski, 2007). Much attention has been drawn to this field of study since the 1970s (Das and Altman, 1972; Bjorklund and Stenevi, 1979) and remarkable successes have been achieved during the last three decades. These studies have shown that neurons in grafted tissue can successfully survive in host brain and develop efferent projections to reestablish synaptic connections between the host and donor neurons (Gaillard and Roger, 2000; Gaillard et al., 2004; Gaillard, 2007; Gaillard et al., 2007; Falkner et al., 2016). Electrophysiological evidence suggests that the grafted neurons develop functional connections in the host cortices of adult animals (Gaillard and Domballe, 2008; Santos-Torres et al., 2009; Jimenez-Diaz et al., 2011) and the data of behavioral tests indicate that the damaged functions can be partially restored after transplantation (Plumet et al., 1993; Riolobos et al., 2001; Gaillard et al., 2007). Our recent study suggests that there is an interactive relationship between the host brain and the transplanted tissue. The transplanted tissue provides neurons to repair the damaged circuit, and host brain can restore the microglial population in the grafted tissue (Wang et al., 2016). However, the survival and differentiation of other essential cell subsets (such as astrocyte and oligodendrocyte) and their roles and functions in the grafted tissue remain undetermined. We hope the approach we described here can be combined with other cutting-edge techniques to reveal the mechanism underlying the reconstructing process between the host brain and transplanted tissue.
Materials and Reagents
- Double-edge razor blade (SHANGHAI RAZOR BLADE, catalog number: 74-s , or Gillette, catalog number: PLATINUM-PLUS® )
- Microsurgical blade (Salvin Dental Specialties, catalog number: 6900 )
- Superglue (cyanoacrylate, Products of ALTECO CHEMICAL, catalog number: SG-12 )
- Gelfoam (Zhejiang AOKI Medical Dressing or Pfizer, catalog number: AZL0009034201 )
- 24-well cell culture plate (Corning, NY)
- 90 mm culture dish (Guangzhou Jet Bio-Filtration, catalog number: TCD010090 )
- Filter paper (Autoclaved)
- Toothpick (Autoclaved)
- Surgical sutures (Yangzhou Jinhuan Medical Apparatus Factory, material: silk, size: 5-0 UPS standard)
- 1 ml Insulin syringe (Shandong Weigao Group Medical Polymer, catalog number: B-D328404Z or BD, catalog number: 328404 )
- 5 ml plastic transfer pipette (Sterilized)
- Mice
Note: Mice of both sexes at the age of 3-4 months are highly recommended to be used as host mice (recipient) in this protocol, and the fetus at the Embryonic day 14 (E14) or E15 (both genders) is used as donor, the strain of mice is depended on the purpose of study. - 75% ethanol (Tianjin Fuyu Fine Chemical)
- Erythromycin ointment (paraffin based lubricant is also recommended)
- Iodine tincture
- Ketamine (Fujian Gutian Parma, catalog number: H35020148 )
- Xylazine (Sigma-Aldrich, catalog number: X1251-1G )
- Sodium chloride (NaCl) (Beichen fangzheng, Tianjin; or Sigma-Aldrich, catalog number: S5886 )
- Potassium chloride (KCl) (Haiguang, Tianjin; or Sigma-Aldrich, catalog number: P5405 )
- Potassium phosphate monobasic (KH2PO4) (The sixth chemical plant, Tianjin; or Sigma-Aldrich, catalog number: P5655 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S9763 )
- Calcium chloride (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902 )
- Magnesium sulfate (MgSO4·7H2O) (Sigma-Aldrich, catalog number: 63138 )
- Na+-HEPES (Sigma-Aldrich, catalog number: H7006 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5767 )
Note: This product has been discontinued. - Glucose
- Urethane (Sigma-Aldrich, catalog number: 94300 )
- Ketamine-Xylazine mixture (KX) (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
- Hanks balanced salt solution (HBSS) (see Recipes)
- Urethane solution (see Recipes)
Equipment
- Dental drill (SEASHIN PRECISION, catalog number: STRONG 90 )
- Curved scissors, cutting edge: 14 mm, material: stainless steel (Fine Science Tools, catalog number: 14084-09 )
- Heating pad (Tme, model: JR-1/2 DC )
- Dissecting microscope (Olympus, model: SZ61 )
- Straight scissors, cutting edge: 14 mm, material: stainless steel (Fine Science Tools, catalog number: 14085-09 )
- Thin-tipped forceps (Fine Science Tools, model: Dumont #5 )
- Straight forceps (VETUS, catalog number: ST-14 )
- Curved forceps (Fine Science Tools, model: Dumont #5/45 )
- Custom-made steel plate (see Figure 1B)
- Compressed air (Sunto, catalog number: ST1005 )
- Biosafety cabinet (Jiangsu Sujing Group, model: BCM-1300A )
- Refrigerator
Software
- ImageJ software (http://rsb.info.nih.gov/ij)
Procedure
- Preparation of host mice (Figure 1A stage 1; Video 1)
Note: Both sexes (male and female) can be used as recipients in this protocol. Embryonic tissue from the fetuses (regardless of their gender) at the Embryonic day 14 (E14) or E15 extracted from the pregnant mouse can be transplanted to ~3 host mice (a success transplantation means the cortical tissue from one embryonic cortex has been transplanted into the lesion cavity of the recipient), and thus ~3 host mice can be prepared.
Figure 1. Experimental procedure of this protocol. A. Diagram showing the surgery procedure for transplantation, including preparation of host mice (stage 1), embryonic brain tissue preparation (stage 2) and transplant the embryonic cortical tissue to host brain (stage 3). At stage 1, an open-skull window was made on the host mouse and a lesion cavity was drilled in the cortex of host brain. At stage 2 embryonic brain was extracted from fetus mice. At stage 3, a piece of embryonic brain tissue was cut from the fetus brain, and grafted into the lesion cavity in adult mouse cortex. B. The host mouse was fixed to the custom-made steel plate. C. The cross-section view of the host brain after transplantation surgery. The open-skull window was covered with the bone flap and glued to the skull using Superglue.Video 1. Preparation of the host mice- Deeply anesthetize the mice with an intraperitoneal injection (100 μl/20 g body weight) of ketamine-xylazine mixture (KX, see Recipes). The anesthetized mice are monitored until the pedal withdrawal response is lost.
- Sterilize all the surgical tools using 75% ethanol and lubricate the mouse’s eyes with erythromycin ointment (or paraffin based lubricant) to protect the eyes from drying during surgery.
- Moisten the hair on mouse head with water and shave the hair over the head with a razor blade.
- Disinfect the shaved scalp with iodine tincture and make an arc-shaped incision on the scalp (Figure 1A stage 1). Separate the scalp and expose the skull. Remove all fascia on the skull with a microsurgical blade.
- Mark the interested region on skull (-2.5 mm bregma, 2.5 mm lateral in this case) and adhere the skull to a custom-made metal frame (made by sticking two double-layered razor blades face to face to each other with Superglue. (A standard stereotactic frame can be also used for this surgery). Make sure the skull has been glued tightly with the metal frame and then fix the frame to a custom-made steel plate tightly (Figure 1B).
Note: The metal frame should not be glued to the skull until the skull was completely dried, otherwise the metal frame will not be able to adhere to the skull tightly. - Use a high-speed dental drill to thin the edge of a circular cranial area (approximately 2 mm in diameter) and clean the skull fragment with compressed air. Drill slowly and prevent the drill bur from puncturing the thinned skull.
Note: Prevent the thinned area from being overheated by adding PBS (at room temperature) frequently. - Remove the bone flap (the separated skull piece) gently with curved forceps after the skull has been thinned enough. Stop any bleeding from the exposed dura with a piece of gelfoam soaked in PBS.
- Clean the bone flap with PBS (see Recipes) and then place it into a 24-well culture plate filled with fresh PBS.
- Choose a cortical region (avoid large vessels) and create a traumatic lesion cavity (about 1 mm in diameter and 1 mm in depth) using the high-speed dental drill with a new disinfected drill bur.
- Clean the tissue debris in the traumatic lesion cavity and blood leaked from the lesion cavity with PBS soaked gelfoam. Detach the metal frame from the mouse skull and stop any further bleeding from the traumatic lesion cavity with gelfoam if necessary.
- Cover the exposed cortex with a piece of gelfoam soaked in PBS to stop bleeding of the lesion cavity. Place the mouse on a heating pad and monitor the state of anesthesia (keep the animal at the surgical level of anesthesia and inject more KX if necessary).
Note: Protect the cortex from desiccation by adding PBS dropwise to the gelfoam frequently.
- Deeply anesthetize the mice with an intraperitoneal injection (100 μl/20 g body weight) of ketamine-xylazine mixture (KX, see Recipes). The anesthetized mice are monitored until the pedal withdrawal response is lost.
- Embryonic brain tissue preparation (Figure 1A stage 2; Video 2)
Note: To maintain the viability of embryonic cortical tissue, try to finish the surgical process (extracting and transplanting) within 20 min. Cortical tissue extracted from the fetus at the Embryonic day 14 (E14) or E15 is suitable for embryonic cortical tissue transplantation.Video 2. Preparation of the embryonic brain tissue- Sterilize all the surgical tools with 75% ethanol, including dissecting microscope, four culture dishes, straight scissors and two thin-tipped forceps, and keep them under the ultraviolet irradiation for 30 min in a biosafety cabinet.
- Prepare four culture dishes and pour 10 ml of 4 °C HBSS (see Recipes) into each culture dish for use.
Note: Keep the HBSS in 4 °C refrigerator before use. - Euthanize the pregnant mouse with a lethal dose of urethane (see Recipes) (about 400 μl) and sterilize with 75% ethanol. Perform an abdomen incision, and then remove the entire uterine horn and transfer it into the prepared culture dish with cold HBSS.
- Cut open the uterine horn and remove the amniotic sac of each embryo. Transfer the embryo into another culture dish.
- Cut off the fetal head, transfer the head to another culture dish with 4 °C HBSS, then peel away the scalp and skull under a dissection microscope. Remove the meninges gently with a pair of forceps to fully expose the embryonic brain. Dissociate the cortical tissue and keep it in another culture dish with cold HBSS.
- Sterilize all the surgical tools with 75% ethanol, including dissecting microscope, four culture dishes, straight scissors and two thin-tipped forceps, and keep them under the ultraviolet irradiation for 30 min in a biosafety cabinet.
- Transplant the embryonic cortical tissue to host brain (Figure 1A stage 3; Video 3)Video 3. Transplant the embryonic cortical tissue to host brain
- Take off the gelfoam on the brain of the host mouse and clear any remaining liquid or blood in the lesion cavity using gelfoam if necessary.
Note: Bleeding from the exposed brain and lesion cavity should be stopped before next step. - Gently pick up the embryonic cortical tissue in the HBSS and carefully cut a piece of embryonic cortical tissue (about 1 mm3) in HBSS with a pair of thin-tipped forceps. Place the tissue into the lesion cavity of the host brain and align the tissue to keep the same orientation as the host brain.
Note: If there is any debris of cortical tissue remains outside of the lesion cavity, clear the debris carefully. - Take out the separated bone flap from 24-well culture plate, and dry it with a piece of autoclaved filter paper and clean any debris on it if necessary.
Note: Ensure to use the same piece of bone flap removed before. - Hold the edge of the bone flap with forceps and cover it lightly to the exposed host brain. Keep it at original orientation and adjust the edge to make it fit well with the cranial window. Stop any bleeding with the gelfoam.
- Wait until the surgical region of the skull is dry. And then apply a small drop of Superglue with a toothpick to seal the seam surrounds the bone flap (Figure 1C).
Note: Care should be taken to prevent the Superglue from infiltrating into the host brain. - Wait ~3 min until the separated skull piece is glued tightly to the skull. Suture the scalp and disinfect it with iodine tincture. Put the mouse back to a separated cage and maintain the animal body temperature at 37 °C until it completely recovers from the anesthesia. The endogenous microglia undergo apoptotic death, and are lost within 36 h after transplantation.
- The survival and differentiation of transplanted tissue can be assessed at different time points from hours to weeks using intravital imaging or fixed tissue techniques. We observed that endogenous microglia of the grafted tissue were lost rapidly after transplantation (starting from 1 h to 36 h after transplantation), and then microglia from host brain infiltrated into the donor tissue at early stage after transplantation (from hours to 1 week). The proliferation of host-derived microglia lasted for at least one month and eventually restored the microglial population in grafted tissue. These data were reported in our recently published article (Scientific Reports 2016, 6:33080). On the other hand, the endogenous neurons of the grafted tissue survived and projected axons and dendrites to the host brain. In Figure 2, we show an example of transplanting a graft from a YFP H-line transgenic fetus to the brain of an adult wild type mouse. In this example, neurons of grafted tissue survived and differentiated in the host brain 2 months after transplantation and projected nerve fibers to the host brain (Figures 2A-2C).
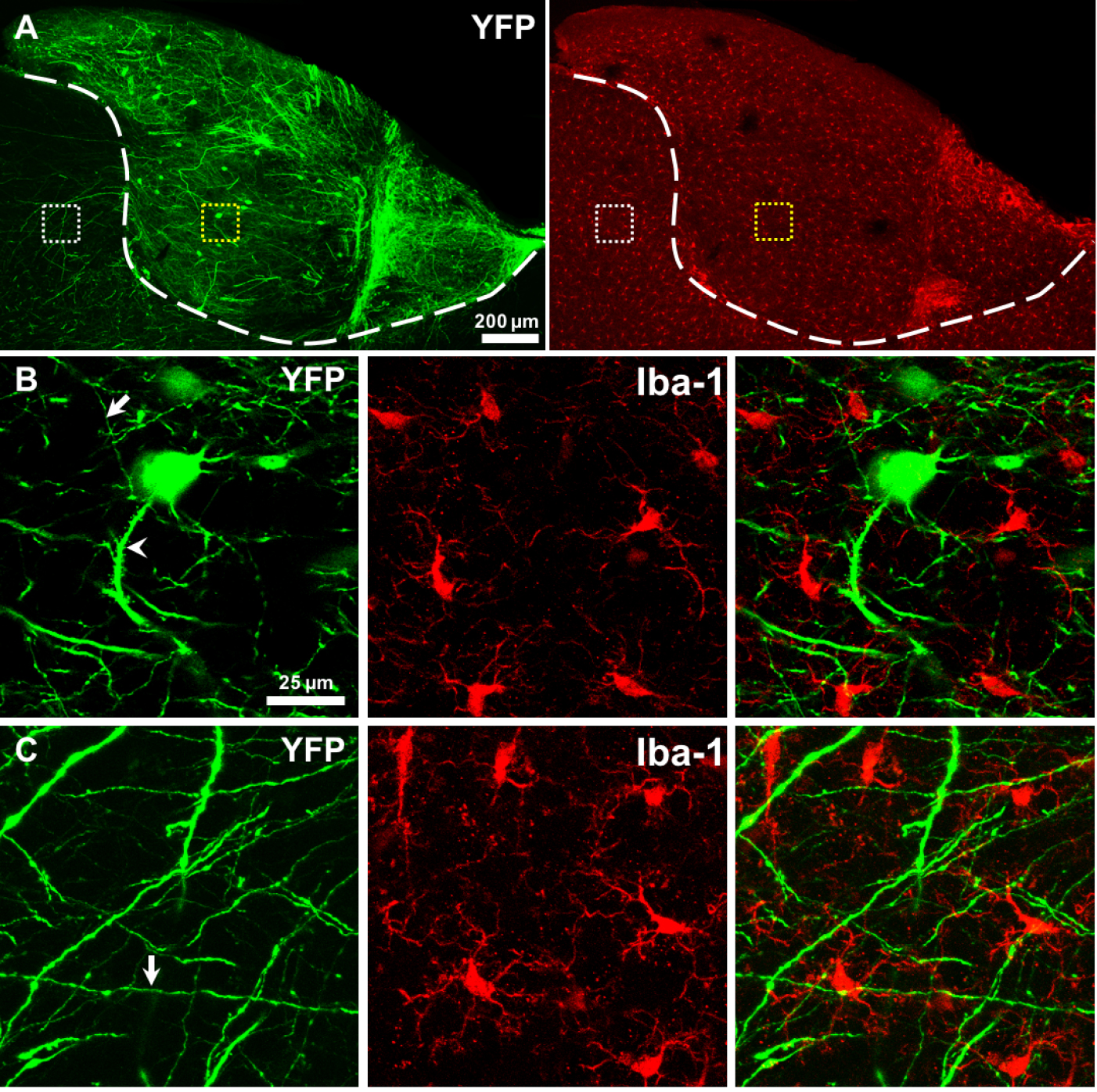
Figure 2. Fetal cortical tissue successful survived and differentiated in host brain. A. Neurons (green) in grafted tissue can survived and differentiated in host brain 2 months after transplantation and projected a multitude of axons and dendrites in the grafted tissue and the host brain. Iba-1 staining showed microglia (red) were uniformly distributed in the grafted tissue and the host brain. The white dash line shows the boundary between donor and recipient tissue. B-C. Magnified view of the yellow and white box regions in A, respectively, showing that neurons projected axons (arrow) and dendrites (arrowhead) and coexisted well with the microglia. The grafted cortical tissues were from a YFP H-line fetus, and the host was a wild-type adult mouse.
- Take off the gelfoam on the brain of the host mouse and clear any remaining liquid or blood in the lesion cavity using gelfoam if necessary.
Data analysis
The development of the grafted tissue was examined by intravital two photon imaging or confocal imaging of fixed brain slices. The differentiation of the grafted tissue was analyzed by evaluating the projection of axons and dendrites of the fluorescently labeled neurons originated from the embryonic tissue. For confocal imaging, the stacks of images were acquired by using an Olympus confocal microscope (FV1000), and the z-projection of each stack of images was performed by using the Z Project function of ImageJ software (http://rsb.info.nih.gov/ij). The images of different channels were merged by using the Merge Channels function of ImageJ.
Notes
- To increase the success rate of the transplantation surgery, the fetuses should be extracted from the euthanatized pregnant mouse as soon as possible.
- During the preparation of donor tissue, the temperature of the embryonic tissue should be kept low by putting them into a culture dish with 4 °C HBSS.
- After the transplantation surgery, each host mouse should be fed in a separate cage to promote the recovery of the host animal. Typical survival rate of the recipient could reach to ~100% if good care has been taken. The success rate of the transplantation surgery is ~55%.
- A circular cover glass instead of the bone flap can be glued to the skull window for intravital two photon imaging.
Recipes
- Ketamine-xylazine mixture (KX)
20 mg/ml ketamine
2 mg/ml xylazine
Dissolved in 0.9% NaCl - Phosphate buffered saline (PBS)
137 mM NaCl
2.7 mM KCl
1.4 mM KH2PO4
8.0 mM Na2HPO4 at pH 7.4
Sterilized and stored in refrigerator - Hanks balanced salt solution (HBSS)
137 mM NaCl
2.5 mM CaCl2
1.0 mM MgSO4
5.0 mM KCl
0.34 mM Na2HPO4
10.0 mM Na+-HEPES
1.0 mM NaHCO3
20.0 mM glucose at pH 7.4
Sterilized and stored in refrigerator - Urethane solution
1.5 g dissolved in 10 m water (ultrapure and sterilized)
Acknowledgments
This study was supported by National Natural Science Foundation of China (Nos. 81171174, 31471045) and Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP: 20130211110002). The method has been used in our recently study (Wang et al., 2016) and it is an adaptation of the methods used in previous publications (Gaillard and Roger, 2000; Gaillard et al., 2007).
References
- Bjorklund, A. and Stenevi, U. (1979). Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res 177(3): 555-560.
- Das, G. D. and Altman, J. (1972). Studies on the transplantation of developing neural tissue in the mammalian brain. I. Transplantation of cerebellar slabs into the cerebellum of neonate rats. Brain Res 38(2): 233-249.
- Falkner, S., Grade, S., Dimou, L., Conzelmann, K. K., Bonhoeffer, T., Gotz, M. and Hubener, M. (2016). Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539(7628): 248-253.
- Gaillard, A., Prestoz, L., Dumartin, B., Cantereau, A., Morel, F., Roger, M. and Jaber, M. (2007). Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci 10(10): 1294-1299.
- Gaillard, A. and Roger, M. (2000). Early commitment of embryonic neocortical cells to develop area-specific thalamic connections. Cereb Cortex 10(5): 443-453.
- Gaillard, F. (2007). Heterotopic, not homotopic, fetal occipital allografts in adult hosts project to visual-related extracortical targets. Restor Neurol Neurosci 25(2): 161-175.
- Gaillard, F. and Domballe, L. (2008). Fetal tissue allografts in the damaged adult visual cortex: physiology and connectivity. Restor Neurol Neurosci 26(4-5): 267-277.
- Gaillard, F., Domballe, L. and Gaillard, A. (2004). Fetal cortical allografts project massively through the adult cortex. Neuroscience 126(3): 631-637.
- Jimenez-Diaz, L., Nava-Mesa, M. O., Heredia, M., Riolobos, A. S., Gomez-Alvarez, M., Criado, J. M., de la Fuente, A., Yajeya, J. and Navarro-Lopez, J. D. (2011). Embryonic amygdalar transplants in adult rats with motor cortex lesions: a molecular and electrophysiological analysis. Front Neurol 2: 59.
- Plumet, J., Ebrahimi, A., Guitet, J. and Roger, M. (1993). Partial recovery of skilled forelimb reaching after transplantation of fetal cortical tissue in adult rats with motor cortex lesion - anatomical and functional aspects. Restor Neurol Neurosci 6(1): 9-27.
- Riolobos, A. S., Heredia, M., de la Fuente, J. A., Criado, J. M., Yajeya, J., Campos, J. and Santacana, M. (2001). Functional recovery of skilled forelimb use in rats obliged to use the impaired limb after grafting of the frontal cortex lesion with homotopic fetal cortex. Neurobiol Learn Mem 75(3): 274-292.
- Santos-Torres, J., Heredia, M., Riolobos, A. S., Jimenez-Diaz, L., Gomez-Bautista, V., de la Fuente, A., Criado, J. M., Navarro-Lopez, J. and Yajeya, J. (2009). Electrophysiological and synaptic characterization of transplanted neurons in adult rat motor cortex. J Neurotrauma 26(9): 1593-1607.
- Tuszynski, M. H. (2007). Rebuilding the brain: resurgence of fetal grafting. Nat Neurosci 10(10): 1229-1230.
- Wang, C., Tao, S., Fang, Y., Guo, J., Zhu, L. and Zhang, S. (2016). Infiltrating cells from host brain restore the microglial population in grafted cortical tissue. Sci Rep 6: 33080.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, C., Gao, H. and Zhang, S. (2017). Transplantation of Embryonic Cortical Tissue into Lesioned Adult Brain in Mice. Bio-protocol 7(12): e2360. DOI: 10.21769/BioProtoc.2360.
Category
Neuroscience > Nervous system disorders > Animal model
Stem Cell > Embryonic stem cell > Cell transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









