- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Expression and Purification of the Cas10-Csm Complex from Staphylococci
Published: Vol 7, Iss 11, Jun 5, 2017 DOI: 10.21769/BioProtoc.2353 Views: 12027
Reviewed by: Lionel SchiavolinLongping Victor TseDaan C. Swarts

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

From Llama to Nanobody: A Streamlined Workflow for the Generation of Functionalised VHHs
Lauren E.-A. Eyssen [...] Raymond J. Owens
Mar 20, 2024 6244 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2095 Views

Thermus thermophilus CRISPR Cas6 Heterologous Expression and Purification
Junwei Wei [...] Yingjun Li
Jul 20, 2025 2171 Views
Abstract
CRISPR-Cas (Clustered regularly interspaced short palindromic repeats-CRISPR-associated proteins) is a class of prokaryotic immune systems that degrade foreign nucleic acids in a sequence-specific manner. These systems rely upon ribonucleoprotein complexes composed of Cas nucleases and small CRISPR RNAs (crRNAs). Staphylococcus epidermidis and Staphylococcus aureus are bacterial residents on human skin that are also leading causes of antibiotic resistant infections (Lowy, 1998; National Nosocomial Infections Surveillance, 2004; Otto, 2009). Many staphylococci possess Type III-A CRISPR-Cas systems (Marraffini and Sontheimer, 2008; Cao et al., 2016), which have been shown to prevent plasmid transfer and protect against viral predators (Goldberg et al., 2014; Hatoum-Aslan et al., 2014; Samai et al., 2015) in these organisms. Thus, gaining a mechanistic understanding of these systems in the native staphylococcal background can lead to important insights into the factors that impact the evolution and survival of these pathogens. Type III-A CRISPR-Cas systems encode a five-subunit effector complex called Cas10-Csm (Hatoum-Aslan et al., 2013). Here, we describe a protocol for the expression and purification of Cas10-Csm from its native S. epidermidis background or a heterologous S. aureus background. The method consists of a two-step purification protocol involving Ni2+-affinity chromatography and a DNA affinity biotin pull-down, which together yield a pure preparation of the Cas10-Csm complex. This approach has been used previously to analyze the effects of mutations on Cas10-Csm complex integrity (Hatoum-Aslan et al., 2014), crRNA formation (Hatoum-Aslan et al., 2013), and to detect binding partners that directly interact with the core Cas10-Csm complex (Walker et al., 2016). Importantly, this approach can be easily adapted for use in other Staphylococcus species to probe and understand their native Type III-A CRISPR-Cas systems.
Keywords: CRISPR-Cas Type III-ABackground
Staphylococcus epidermidis and Staphylococcus aureus are prevalent skin-dwelling bacteria that have a range of opposing impacts. While S. aureus asymptomatically colonizes ~30% of the population (Conlan et al., 2012), this organism is a leading cause of skin and soft tissue infections (Stryjewski and Chambers, 2008; Grice and Segre, 2011). In contrast, S. epidermidis is generally considered beneficial, and promotes human health by 1) preventing S. aureus colonization (Iwase et al., 2010), 2) producing antimicrobial peptides that target skin pathogens (Cogen et al., 2010), and 3) stimulating the human immune system to facilitate pathogen defense (Lai et al., 2010; Naik et al., 2015). However, when allowed to breach the skin barrier, this species can also cause antibiotic resistant infections, particularly on indwelling medical devices (Otto, 2009; Harris and Richards, 2006). Furthermore, pathogenic staphylococci that are resistant to all known antibiotics have recently emerged in both hospital and community settings (Furuya and Lowy, 2006) and have become a major threat to global public health. Horizontal gene transfer (HGT), or the exchange of genetic information between related bacterial species, is a major route by which these organisms acquire virulence factors and multi-drug resistance. Therefore, it is of utmost importance to understand the factors that impact and regulate HGT in these organisms.
CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR associated proteins) is a class of bacterial immune systems that degrade invading nucleic acids and prevent all modes of HGT (Marraffini, 2015). CRISPR loci consist of short sequences derived from past invaders, known as spacers, which are integrated between repeat sequences of similar length (~30-40 nucleotides). These repeat-spacer arrays encode small CRISPR RNAs (crRNAs) that associate with Cas proteins, forming a ribonucleoprotein complex that destroys foreign DNA and/or RNA in a sequence-dependent manner. Many staphylococci possess Type III-A CRISPR-Cas systems (Marraffini and Sontheimer, 2008; Golding et al., 2012; Cao et al., 2016). The Type III-A system in S. epidermidis RP62a, a wild-type human isolate (Christensen et al., 1987), encodes a multi-subunit complex called Cas10-Csm, composed of Cas10, Csm2, Csm3, Csm4, Csm5 and a crRNA (Hatoum-Aslan et al., 2013). This system has been shown to prevent the conjugative transfer of antibiotic resistance genes (Marraffini and Sontheimer, 2008; Hatoum-Aslan et al., 2014) and phage infection (Goldberg et al., 2014; Maniv et al., 2016), thus providing a natural barrier for HGT, and a model for Type III CRISPR-Cas systems in staphylococci.
The overexpression and purification of recombinant CRISPR-associated proteins from Escherichia coli (both the Cas10-Csm complex and individual subunits) followed by in vitro biochemical assays have revealed important insights into their functions (Hatoum-Aslan et al., 2013; Samai et al., 2015; Walker et al., 2016). However, such assays fail to 1) recover information about protein function and stability in the native cellular environment, and 2) identify biologically relevant binding partners that are not a part of the core Cas10-Csm complex. Indeed, purification of Cas10-Csm from its native S. epidermidis background has yielded additional insights into the genetic requirements for complex stability and function, crRNA processing, and non-Cas binding partners that might play a role in the CRISPR-Cas pathway (Hatoum-Aslan et al., 2013 and 2014; Walker et al., 2016). Here, we provide a detailed protocol for the purification of Cas10-Csm from S. epidermidis or S. aureus strains bearing the Type III-A CRISPR-Cas system on a plasmid. The protocol involves two affinity-purification steps that can be carried out over the course of five days (Figure 1). Importantly, this protocol can be easily adapted to study Cas10-Csm complexes in other Staphylococcus species, thus providing an essential tool to probe and understand these important immune systems.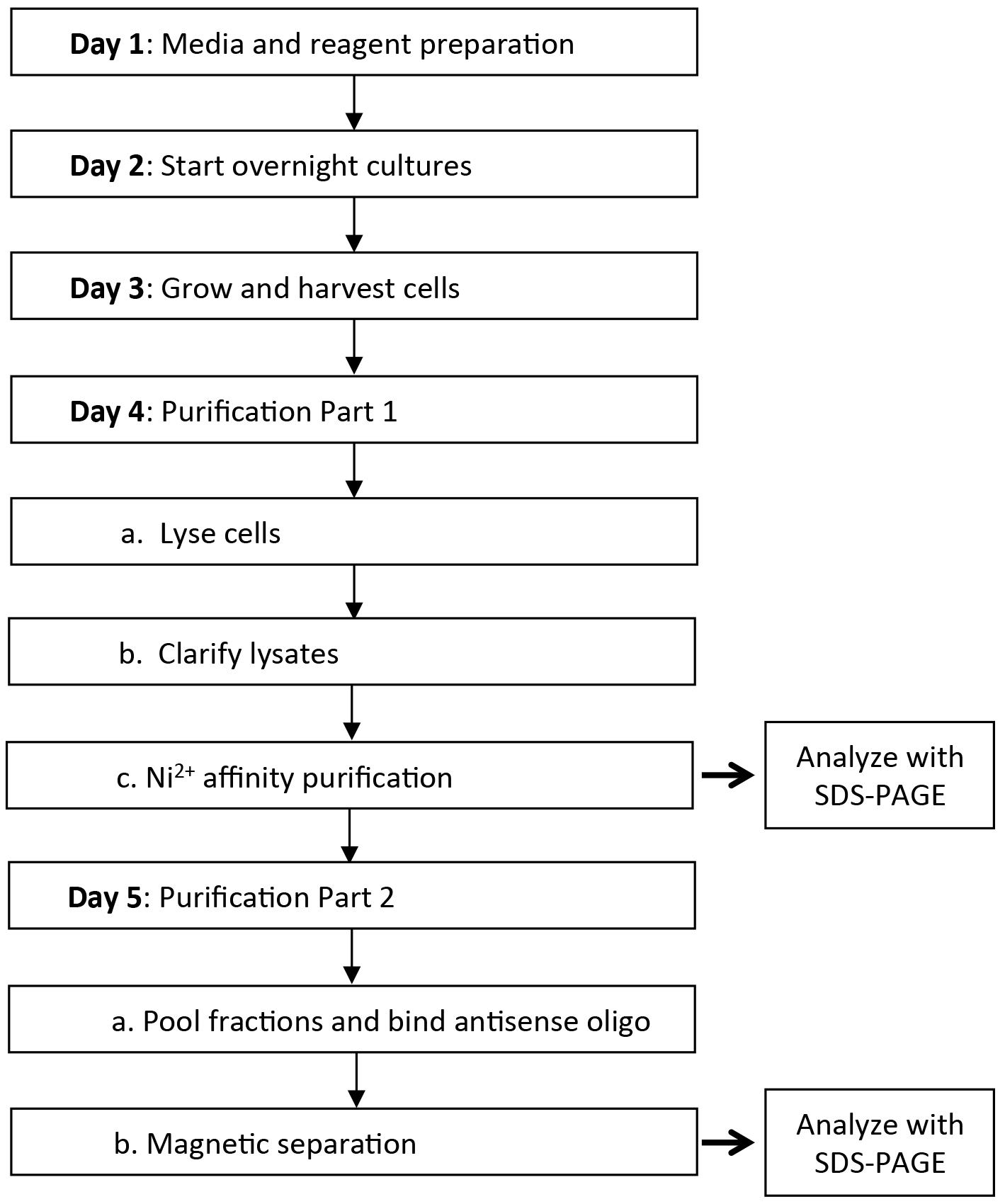
Figure 1. Timeline of activities for expression and purification of the Cas10-Csm complex from staphylococci
Materials and Reagents
Note: Equivalent materials and reagents may be used as substitutes.
- Centrifuge tubes (50 ml) (VWR, catalog number: 21008-242 )
- Pipet tips with filter (0.1-10 µl) (VWR, catalog number: 89368-972 )
- Pipet tips with filter (1-200 µl) (VWR, catalog number: 89003-056 )
- Pipet tips with filter (100-1,000 µl) (VWR, catalog number: 89003-060 )
- PES membrane vacuum filter (0.22 µm) (VWR, catalog number: 10040-468 )
- Centrifuge tubes (15 ml) (VWR, catalog number: 21008-216 )
- Microcentrifuge tubes (VWR, catalog number: 87003-294 )
- Cellulose syringe filter (0.22 µm) (VWR, catalog number: 28145-477 )
- Petri dishes (100 x 15 mm) (VWR, catalog number: 25384-088 )
- Spectrophotometer cuvettes (VWR, catalog number: 97000-586 )
- Syringe (10 ml) (BD, catalog number: 309604 )
- Syringe (3 ml) (BD, catalog number: 309657 )
- Centrifugation polypropylene bottles (400 ml) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 75007585 )
- Disposable gravity flow columns for protein purification (Geno Technology, G-Biosciences, catalog number: 786-169 )
- S. epidermidis LM1680 expressing pcrispr/Csm26HN (Hatoum-Aslan et al., 2013) (see Note 1)
- S. aureus RN4220 expressing pcrispr/Csm26HN (Hatoum-Aslan et al., 2013) (see Note 1)
- HisPur Ni-NTA resin (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88222 )
- Sera-MagTM magnetic streptavidin coated beads (GE Healthcare, catalog number: 30152105011150 )
- SDS PAGE Gel 0.75 MM ‘Snap-A-GelsTM Mini Tris Glycine Precast Gels, Jule’ (VWR, catalog number: 66025-389 )
- Color protein standard ladder (New England Biolabs, catalog number: P7712S )
- BBLTM brain heart infusion (BHI) broth (BD, BBLTM, catalog number: 211060 )
- DifcoTM brain heart infusion (BHI) agar (BD, DifcoTM, catalog number: 241810 )
- Chloramphenicol (Alfa Aesar, catalog number: B20841 )
- 100% ethanol (Decon Labs, catalog number: V1016TP )
- Neomycin sulfate (AMRESCO, catalog number: 0558-25G )
- Magnesium chloride (MgCl2) (AMRESCO, catalog number: J364 )
- Tris (AMRESCO, catalog number: 0497 )
- Hydrochloric acid (HCl) (VWR, BDH®, catalog number: BDH3030-2.5LPC )
- Potassium chloride (KCl) (VWR, BDH®, catalog number: BDH9258-500G )
- EDTA, disodium salt, dihydrate (EMD Millipore, OmniPur®, catalog number: 4050 )
- Sodium hydroxide (NaOH) (AMRESCO, catalog number: 0583 )
- Sodium dodecyl sulfate (SDS) (VWR, catalog number: 97064-862 )
- Bromophenol blue (VWR, catalog number: 97061-690 )
- Ambicin® L (Recombinant lysostaphin) (AMBI, catalog number: LSPN-50 )
- Sodium acetate anhydrous (NaOAc) (AMRESCO, catalog number: 0602 )
- Sodium phosphate monobasic (NaH2PO4) (AMRESCO, catalog number: 0571 )
- Sodium chloride (NaCl) (VWR, BDH®, catalog number: BDH9286 )
- Glycerol (AMRESCO, catalog number: M152 )
- Beta-mercaptoethanol (β-ME) (Geno Technology, G-Biosciences, catalog number: BC98 )
- Coomassie Blue G-250 (AMRESCO, catalog number: M140-10G )
- Methanol (VWR, BDH®, catalog number: BDH20864.400 )
- Acetic acid (HAc) (VWR, BDH®, catalog number: BDH3096-2.5LPC )
- Tris-glycine-SDS 10x buffer (AMRESCO, catalog number: 0783-5L )
- Imidazole (Alfa Aesar, catalog number: A10221 )
- Pierce protease and phosphatase inhibitor mini tablets, EDTA-free (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88669 )
- Triton X-100 surfactant (EMD Millipore, catalog number: TX1568 )
- Potassium hydroxide (KOH) (Alfa Aesar, catalog number: 13451 )
- Media and antibiotics (see Recipes)
- Brain heart infusion (BHI) broth
- Chloramphenicol stock (10 mg/ml)
- Neomycin stock (15 mg/ml)
- Stock solutions (see Recipes)
- 1 M MgCl2
- 1 M Tris-HCl, pH 8.3
- 1 M Tris-HCl, pH 6.8
- 1 M KCl
- 500 mM EDTA, pH 8.0
- 10% sodium dodecyl sulfate (SDS)
- 1% bromophenol blue
- 2 mg/ml recombinant lysostaphin
- 2x lysis buffer
- 5x annealing buffer
- 5x protein loading buffer
- Coomassie Blue staining solution
- Destaining solution
- 1x Tris-glycine buffer
- Buffers to prepare on Day 4 for purification–Part 1 (see Recipes)
- Elution buffer
- Resuspension buffer
- Equilibration buffer
- Wash 1 buffer
- Wash 2 buffer
Equipment
- Eppendorf Research® plus pipettes set (Eppendorf, catalog number: 2231000222 ), or equivalent pipettes set with a range of 1 μl to 1,000 μl
- Media/storage bottles (250 ml) (VWR, catalog number: 10754-816 ), or equivalent
- Erlenmeyer flask (2 L) (VWR, catalog number: 10545-844 ), or equivalent
- Standard orbital shaker (VWR, model: 1000, catalog number: 89032-088 ), or equivalent shaker that can gently rotate for gel staining and destaining
- New Brunswick I26 incubating shakers (Eppendorf, New BrunswickTM, model: I26 , catalog number: M1324-0008), or equivalent shaking incubator that can maintain 37 °C and 180 rpm
- Heraeus Multifuge X1R centrifuge series (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM MultifugeTM X1R , catalog number: 75004251), or equivalent refrigerated centrifuge with a rotor capable of holding 400 ml bottles and applying 13,000 x g of centrifugal force
- General purpose water baths (VWR, model: VWR General Purpose Water Baths, catalog number: 89501-460 ), or equivalent capable of maintaining 37 °C
- Sonifier® S-450 Analog sonicator (Emerson, Branson, model: S-450 , catalog number: 101-063-198), or equivalent ultrasonic homogenizer with operating frequency of 20 kHz and tip diameter of 3.2 mm
- 30 ml beaker (Corning, PYREX®, catalog number: 1000-30 ) or equivalent
- Digital dry block heaters (VWR, catalog number: 12621-088 ), or equivalent block heater capable of heating up to 95 °C
- Magnetic bead separation rack (Thermo Scientific, catalog number: MR02 ), or equivalent rack capable of collecting magnetic beads
- Autoclave (Getinge), or equivalent autoclaving instrument capable of heating up to 121 °C while applying 15 atm pressure
- Graduated cylinder (100 ml) (VWR, catalog number: 65000-006 ), or equivalent
- Graduated cylinder (1 L) (VWR, catalog number: 65000-012 ), or equivalent
- Media/storage bottles (1 L) (VWR, catalog number: 10754-820 ), or equivalent
- Media/storage bottles (100 ml) (VWR, catalog number: 10754-814 ), or equivalent
- Media/storage bottles (500 ml) (VWR, catalog number: 10754-818 ), or equivalent
- UltrospecTM 10 cell density meter (GE Healthcare, catalog number: 80-2116-30 ), or equivalent spectrophotometer that can measure the density of cells in suspension at 600 nm
- Mini-PROTEAN tetra cell (Bio-Rad Laboratories, model: Mini-PROTEAN Tetra Cell, catalog number: 1658004EDU ), or equivalent vertical electrophoresis system
- pH meter AccumetTM AB15 plus basic (Fisher Scientific, model: Fisher ScientificTM accumetTM AB15+ Basic and BioBasicTM, catalog number: 13-636-AB15P ), or equivalent pH meter with a range of 0.000-10.000
Procedure
- Day 1. Preparation of media, reagents, and single colonies
- Prepare 100 ml of brain heart infusion (BHI) broth in a 250-ml bottle (for starting overnight cultures), and 1 L of BHI broth in a 2 L Erlenmeyer flask (for large-scale propagation and protein purification) (see Recipe 1).
- Prepare antibiotics listed in Recipe 1.
- Prepare all the stock solutions needed for the experiment (see Recipe 2).
- Streak out a freezer stock of S. epidermidis LM1680 bearing pcrispr/Csm26HN (or S. aureus RN4220 bearing pcrispr/Csm26HN) on a BHI agar plate containing 10 μg/ml chloramphenicol. If S. epidermidis is used, supplement media with 15 μg/ml neomycin (see Note 1).
- Prepare 100 ml of brain heart infusion (BHI) broth in a 250-ml bottle (for starting overnight cultures), and 1 L of BHI broth in a 2 L Erlenmeyer flask (for large-scale propagation and protein purification) (see Recipe 1).
- Day 2. Overnight culture preparation
- Transfer 10 ml of BHI broth into a 50-ml conical tube.
- Add 10 μl of 10 mg/ml chloramphenicol to select for pcrispr/Csm26HN. If S. epidermidis LM1680 is being used, add additionally 10 μl of 15 mg/ml neomycin. If other strains and plasmids are being used, supplement media with antibiotics as appropriate (see Note 1).
- With a sterile pipette tip, pick a single colony of S. epidermidis LM1680 bearing pcrispr/Csm26HN (or S. aureus RN4220 bearing pcrispr/Csm26HN).
- Re-suspend the colony into the 10 ml of autoclaved media containing appropriate antibiotic(s).
- Close loosely the 50-ml conical tube containing the autoclaved media, antibiotics, and colony of interest.
- Incubate the bacterial culture at 37 °C for 16-20 h in a shaking incubator set to 180 rpm.
- Transfer 10 ml of BHI broth into a 50-ml conical tube.
- Day 3. Large-scale propagation of cells overexpressing the Cas10-Csm complex
- Into the 1 L of BHI prepared in the 2 L Erlenmeyer flask, add 1 ml of 10 mg/ml chloramphenicol. If S. epidermidis LM1680 is being used, add additionally 1 ml of 15 mg/ml neomycin.
- Inoculate the 1 L of BHI plus antibiotic(s) with the entire 10 ml of overnight culture prepared on Day 2.
Note: The OD600 nm for overnight S. epidermidis and S. aureus cultures is approximately 5-6. - Measure the initial OD600 nm of diluted culture with a cell density meter to facilitate estimation of time required to reach the final OD600 nm (see below).
- Incubate the bacterial culture at 37 °C in a shaking incubator set to 180 rpm.
- Let the bacterial culture grow until OD600 nm = 2.00 (approximately 7-9 h if starting with a fresh overnight culture and initial OD600 nm = 0.05).
- Distribute the 1 L of liquid culture into four 400-ml polypropylene bottles.
- Centrifuge cells at 5,000 x g, 4 °C for 10 min.
- Discard supernatant and resuspend each pellet on ice with 10 ml of ice-cold distilled H2O (dH2O).
- Combine cell suspensions from all pellets into a clean 50 ml conical tube (on ice) and centrifuge at 5,000 x g, at 4 °C for 10 min.
- Discard supernatant and store pelleted cells in -80 °C (for optimal purification up to a week later) or proceed directly to purification.
- Into the 1 L of BHI prepared in the 2 L Erlenmeyer flask, add 1 ml of 10 mg/ml chloramphenicol. If S. epidermidis LM1680 is being used, add additionally 1 ml of 15 mg/ml neomycin.
- Day 4. Purification–Part 1
- Thaw the cell pellet on ice for 1 h.
- Prepare all buffers described in Recipe 3.
- Resuspend thawed pellet in 9 ml of ice-cold dH2O.
- Add 200 µl of 1 M MgCl2 and 125 µl of freshly thawed lysostaphin (2 mg/ml stock).
- Incubate the cell suspension for 1 h in a 37 °C water bath. Invert the tube several times after 30 min of incubation to homogenize the suspension.
- Add 10 ml of resuspension buffer to the lysed cells and invert tube several times. The cell lysate will become very viscous (see Figure 2).
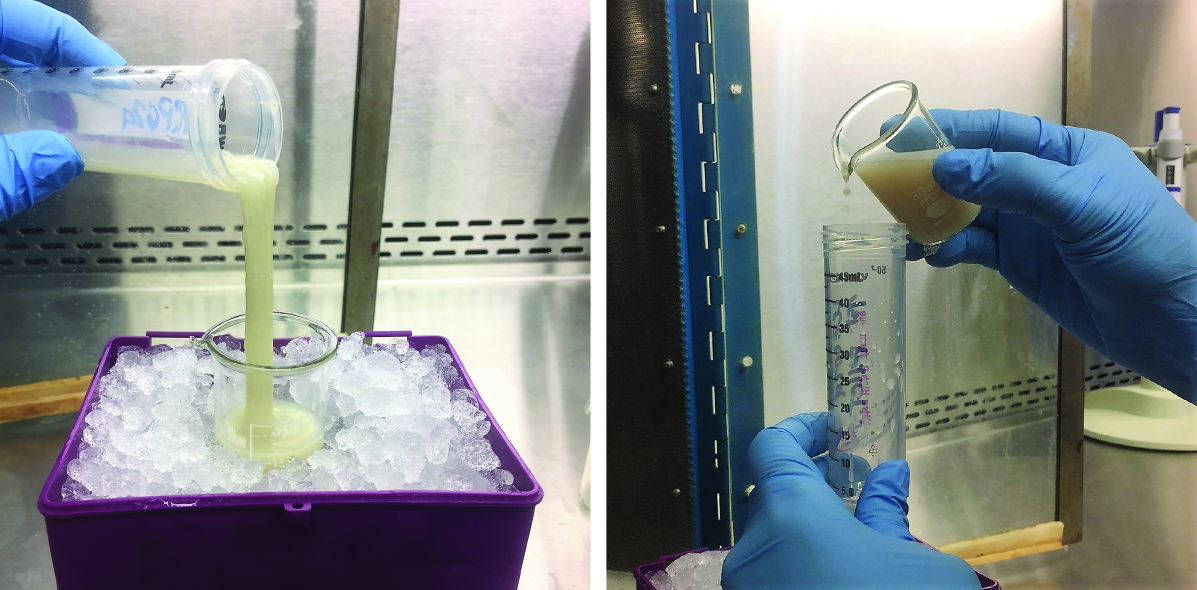
Figure 2. Viscosity of cell lysate before and after sonication of S. epidermidis LM1680 cells expressing pcrispr/Csm26HN. Before sonication (left), the cell lysate is milky white with the consistency of mucus. After sonication (right), the cell lysate becomes tinted brown with a more watery consistency.
- Adjust the Sonifier S-450 to power level 6.0 and duty cycle percent to 6.
Note: These settings correspond to a 60 W power output with a fixed repetition rate of 1 pulse per second. A duty cycle of ‘6’ indicates that power will be delivered 60% of each 1 sec pulse (with a rest 40% of each second). - Pour the lysate into a 30-ml beaker set in an ice bucket. The cell lysate should occupy ~⅔ of the beaker’s volume such that lysate within has at least 1 inch depth.
- Immerse the sonicator tip into the lysate at a depth of ~½-⅔ inch such that there remains ~½ an inch of clearance from the bottom of the beaker.
Important Note: The tip should not touch the sides or bottom of the beaker. - Sonicate the lysate on ice for 30 sec at a time with one minute rest period in between. This cycle should be repeated three times or until the lysate has a more watery consistency. Often, the lysate will also acquire a brown tint (see Figure 2).
- Pour the lysate back into a 50-ml conical tube and centrifuge for 20 min, 4 °C, at 10,000 x g. The clarified lysate after centrifugation often retains the brownish tinge (see Figure 3).

Figure 3. Appearance of sonicated cell lysate following the first centrifugation. After the cell debris is pelleted, the clarified lysate retains a yellow-brown tinge. - Decant the supernatant into a fresh 50-ml conical tube and centrifuge for 20 min, 4 °C, at 13,000 x g.
Note: The cell pellet may be saved and checked later on SDS-PAGE for the presence of the Cas10-Csm complex to assess its solubility. - During the two spins, prepare/pack the column (to be done in the cold room or inside a deli refrigerator):
- Pipet 1 ml of slurry Ni-NTA resin into a disposable gravity flow column and allow the liquid to flow through.
- Equilibrate the Ni-NTA resin by passing 10 ml of equilibration buffer through the column at 4 °C.
- Cap the column once most of the buffer has passed through and ~2-3 mm of buffer remains atop the resin.
- Pipet 1 ml of slurry Ni-NTA resin into a disposable gravity flow column and allow the liquid to flow through.
- After the final spin (step D12), pass the lysate through a 0.22 µm PES membrane vacuum filter and catch the filtrate into a sterile bottle.
- Pass the filtered lysate through the packed Ni-NTA column (prepared in step D13) and collect the flow through in a 50-ml conical tube labeled ‘lysate flow-through’.
Note: The lysate flow-through can be checked later on SDS-PAGE for the presence of the Cas10-Csm complex in case the complex was suspected to have passed through the column without binding. - Wash the column by passing 10 ml of wash 1 buffer through the column.
- Collect wash 1 flow-through into a 15-ml conical tube.
Note: The wash 1 flow-through may be saved and checked later for the Cas10-Csm complex in case the complex was suspected to have passed through the column during the wash. - Wash the column a second time by passing 10 ml of wash 2 buffer through the column.
- Collect wash 2 flow-through into a 15-ml conical tube.
Note: The wash 2 flow-through may be saved and checked later for the Cas10-Csm complex in case the complex was suspected to have passed through the column during the wash. - Elute proteins from the column with 5-7 aliquots of elution buffer (500 µl for each fraction). As each aliquot flows through, capture it into a fresh, appropriately-labeled microcentrifuge tube.
- Into fresh labeled microcentrifuge tubes, combine 20 µl of each fraction with 5 µl of 5x protein loading buffer.
- Heat samples at 95 °C for 5 min on a heating block.
- Load samples alongside a protein standard into a 12% SDS-PAGE gel.
- Run the gel in Tris-glycine-SDS 1x buffer at 120 V for 1 h.
- Stain the gel with Coomassie Blue solution for 10 min and destain with destaining solution for up to 40 min.
- Image the gel under white light (Figure 4).
- Save the desired elutions at -20 °C (see Note 2).
Note: Avoid repeated freeze-thaw cycles of the complex as this leads to protein aggregation. If necessary, aliquot the desired elutions into smaller volumes (~100 µl). Protein complexes that have not been repeatedly thawed can be stored in the elution buffer up to a year at -20 °C without noticeable degradation of protein or crRNAs.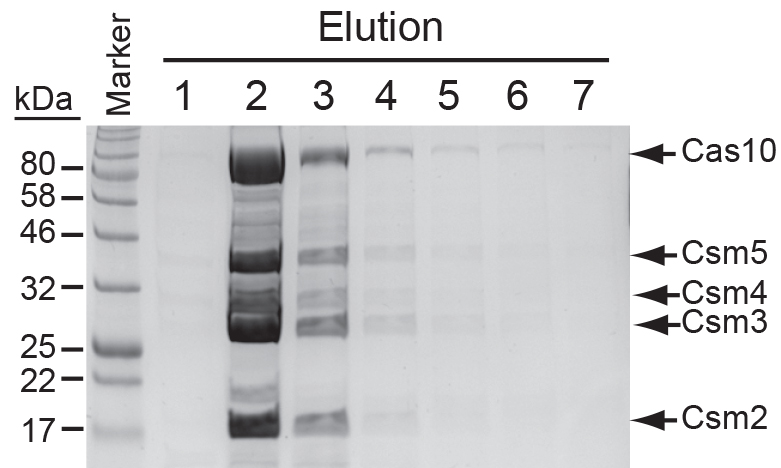
Figure 4. Elutions of the Cas10-Csm complex following purification from S. epidermidis LM1680 expressing pcrispr/Csm26HN. Shown are the seven fractions (E1-E7) collected from a nickel-agarose column and analyzed on a 12% SDS PAGE gel. Proteins were stained with Coomassie Blue G-250.
- Thaw the cell pellet on ice for 1 h.
- Day 5. Purification–Part 2
- Pipette 10 µl of Sera-MagTM magnetic streptavidin coated beads into a microcentrifuge tube.
Note: Mix the bead suspension well by pipetting up and down several times before use. - Resuspend the beads with 100 µl of 1x annealing buffer (see Recipe 2).
Note: Bead re-suspension is achieved once the solution becomes a homogenous brown color and no beads accumulation is seen at the side or bottom of the tube. When tubes are removed from the magnetic rack, the beads within can be easily resuspended. - Pellet the beads by placing the microcentrifuge tube into a magnetic separation rack and waiting for the beads to collect on the side of the tube.
Note: The collection process can vary from 30 sec up to 2 min depending on beads and rack used. The collection process will be completed once the supernatant becomes clear and the beads (brown color) aggregate on the side of the tube where the magnet is located. If the beads are broadly spread around the side of the tube, twist the microcentrifuge tube a few degrees clockwise, or counter-clockwise within the rack to pull all beads closer together. - Gently pipette out the supernatant without disturbing the beads.
- Repeat the wash (steps E2-E4) three times.
- Pool the most concentrated Cas10-Csm complex fractions (obtained in Day 4) and combine with 2 ng of a 5’-biotinylated oligonucleotide antisense to a crRNA (see Note 3).
- Let the mixture incubate for 30 min at room temperature.
- Remove the microcentrifuge tube that contains the beads away from the magnetic rack and add the Cas10-Csm complex/oligo mixture into the equilibrated streptavidin beads.
- Resuspend beads until the mixture appears homogenous and let it anneal at room temperature for 30 min.
- Collect beads to the side of the tube by placing the microcentrifuge tube back into the magnetic rack ~30 sec.
- Gently pipette out the supernatant without disturbing the beads.
- Remove tube from the magnetic rack and resuspend the beads in 100 µl of 1x annealing buffer.
- Collect beads to the side of the tube by placing the microcentrifuge tube back into the magnetic rack ~30 sec.
- Gently pipette out the supernatant without disturbing the beads.
- Repeat the wash (steps E12-E14) two more times.
- Add 20 µl of 1x annealing buffer to the beads and 5 μl of 5x protein loading buffer.
- Heat the samples for 5 min at 95 °C on a heating block.
- Place the tube back into the magnetic rack to remove the beads from solution.
- Load the supernatant alongside a protein standard into a 12% SDS-PAGE gel.
- Run the gel in Tris-glycine-SDS 1x buffer at 120 V for 1 h.
- Stain the gel with Coomassie Blue solution for 10 min and destain with destaining solution for up to 40 min.
- Image the gel under white light (Figure 5).
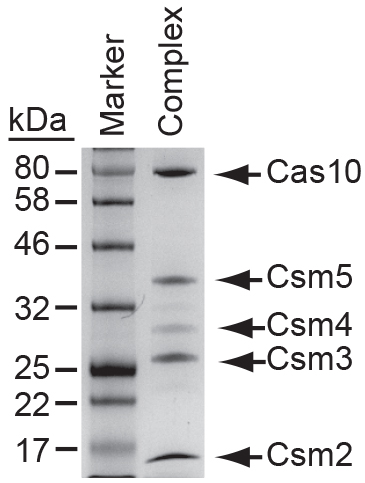
Figure 5. S. epidermidis Cas10-Csm complex collected from magnetic streptavidin beads following an affinity purification with a biotinylated oligonucleotide antisense to spc1 crRNAs. Proteins were resolved on a 12% SDS PAGE gel and visualized with Coomassie Blue G250.
- Pipette 10 µl of Sera-MagTM magnetic streptavidin coated beads into a microcentrifuge tube.
Data analysis
This assay is used as a qualitative measure to assess Cas10-Csm complex integrity. The presence or absence of a particular protein subunit on the SDS-PAGE gel will indicate whether or not it is stably associated with the complex. Using this protocol, the presence or absence and lengths of crRNAs within the complex can be determined by extracting crRNAs directly from Cas10-Csm complex aliquots obtained from the 1st purification process, followed by 5’-end labeling with γ-32P-ATP, and resolving these on an 8-12% polyacrylamide gel. Before a definitive determination of complex stability can be made, these assays must give consistent results over three independent replicates. For complexes that are being characterized for the first time, the identity of each subunit in the gel must be confirmed using Western blotting. These additional methods are described in (Hatoum-Aslan et al., 2013).
Notes
- This protocol describes the purification of Cas10-Csm complexes from S. epidermidis LM1680 or S. aureus RN4220 cells bearing pcrispr/Csm26HN, a plasmid that encodes the entire Type III-A CRISPR-Cas system from S. epidermidis RP62a with a 6x-His tag introduced into the N-terminus of csm2 (Hatoum-Aslan et al., 2013). S. epidermidis LM1680 is a derivative of S. epidermidis RP62a with a chromosomal deletion encompassing the CRISPR-Cas locus (Hatoum-Aslan et al., 2013). S. aureus RN4220 is a crispr-strain (Nair et al., 2011) that is used as a cloning intermediate. When re-introduced on pcrispr/Csm2H6N, the CRISPR-cas system retains full functionality in both strains. pcrispr/Csm2H6N requires selection in 10 µg/ml of chloramphenicol. Additionally, 15 µg/ml of neomycin is used to select specifically for S. epidermidis RP62a or LM1680. This protocol can also be adapted to overexpress the CRISPR-Cas system on different plasmids, or in different strains, however, antibiotic selection would also have to be modified accordingly. If working with wild-type strains, overexpressing a plasmid encoding the CRISPR-Cas system with one subunit 6x-His tagged should, in theory, allow for Cas10-Csm complex purification even in the presence of the chromosomal copy of the system in the background.
- Mass spectrometry analysis may be performed following the first purification to detect potential binding partners loosely associated with the complex. In addition, the presence or absence of crRNAs within complexes and their lengths can be determined by extracting RNAs from complexes following the first purification, end-labeling the RNAs, and resolving on a urea PAGE gel. If a cleaner prep is desired or to assess complex stability, the second purification described for day 5 should be performed.
- The sequence of the biotinylated oligonucleotide used in this example is complementary to the first spacer (spc1) of the repeat-spacer array:
5’-Biotin-TEG-ACGAGAACACGUAUGCCGAAGUAUAUAAAUC
Recipes
- Media and antibiotics
- Brain heart infusion (BHI) broth
- Dissolve 3.7 g per 100 ml dH2O
- Autoclave at 121 °C for 30 min
- Store at room temperature
- Chloramphenicol stock (10 mg/ml)
- Dissolve 100 mg chloramphenicol into 10 ml of 100% ethanol
- Pass through a 0.22 μm syringe filter
- Store at 4 °C
- Neomycin stock (15 mg/ml)
- Dissolve 150 mg neomycin into 10 ml dH2O
- Pass through a 0.22 μm syringe filter
- Store at 4 °C
- Stock solutions
- 1 M MgCl2
- Place 9.521 g MgCl2 into a 100-ml graduated cylinder
- Fill with dH2O up to 100 ml
- Store in a bottle at room temperature
- 1 M Tris-HCl, pH 8.3
- Place 121.14 g Tris into a 1-L graduated cylinder
- Dissolve with 800 ml of dH2O
- Adjust pH to 8.3 with 12 N HCl
- Fill with dH2O up to 1 L
- Store in a bottle at room temperature
- 1 M Tris-HCl, pH 6.8
- Place 121.14 g Tris into a 1-L graduated cylinder
- Dissolve with 800 ml of dH2O
- Adjust pH to 6.8 with 12 N HCl
- Fill with dH2O up to 1 L
- Store in a bottle at room temperature
- 1 M KCl
- Place 7.455 g KCl into a 100-ml graduated cylinder
- Fill with dH2O up to 100 ml
- Store in a bottle at room temperature
- 500 mM EDTA, pH 8.0
- Place 93.06 g EDTA, disodium salt, dihydrate (MW = 372.24 g/mol) into a 500-ml beaker
- Dissolve as much as possible with 350 ml of dH2O
- Adjust pH to 8.0 with 10 N NaOH while constantly stirring
Note: The EDTA will not dissolve completely unless its pH is at 8.0. However, the pH of EDTA continually drops as it gets dissolved. Therefore, constant addition of 10 N NaOH must be used to get the EDTA completely dissolved with a final pH of 8.0. - Fill with dH2O up to 500 ml
- Store in a bottle at room temperature
- 10% sodium dodecyl sulfate (SDS)
- Place 1 g of SDS into a 15-ml conical tube
- Dissolve with 6 ml of dH2O
- Fill with dH2O up to 10 ml
- Store at room temperature
Note: Since powdered SDS can cause serious eye and lung damage, a 20% solution may be purchased (VWR Cat. No. 97062-440) and diluted with dH2O (1:1).
- Place 1 g of SDS into a 15-ml conical tube
- 1% bromophenol blue
- Place 0.1 g of bromophenol blue into a 15-ml conical tube
- Fill with dH2O up to 10 ml
- Store at room temperature
- 2 mg/ml recombinant lysostaphin
- Dissolve 50 mg lysostaphin in 25 ml NaOAc, pH 4.5
- Distribute 0.5 ml aliquots into microcentrifuge tubes
- Store at -80 °C
- Thaw just prior to use
- 2x lysis buffer
- Place 11.998 g NaH2PO4 into a 1-L graduated cylinder ([final] = 100 mM)
- Place 35.064 g NaCl into the graduated cylinder ([final] = 600 mM)
- Fill to 800 ml with dH2O
- Adjust pH to 8.0 with 10 N NaOH
- Fill up to 1 L
- Store at room temperature
- 5x annealing buffer
- Place 2.5 ml of 1 M Tris-HCl, pH 8.3 into a 100-ml bottle ([final] = 25 mM)
- Place 37.5 ml of 1 M KCl into the bottle ([final] = 375 mM)
- Place 1 ml of 500 mM EDTA, pH 8.0 into the bottle ([final] = 5 mM)
- Add 59 ml of dH2O
- Store bottle at room temperature
- 5x protein loading buffer
- Place 0.5 ml of 1 M Tris-HCl, pH 6.8 into a 15-ml conical tube ([final] = 62.5 mM)
- Place 0.8 ml of 100% glycerol into the tube ([final] = 10%)
- Place 1.6 ml of 10% SDS solution into the tube ([final] = 2%)
- Place 0.4 ml of 14.3 M β-mercaptoethanol ([final] = 715 mM)
- Add 0.4 ml of 1% bromophenol blue into the tube ([final] = 0.05%)
- Add 4.3 ml of dH2O
- Distribute 1 ml aliquots into microcentrifuge tubes
- Store at -20 °C
- Coomassie Blue staining solution
- Place 1 g of Coomassie Blue G-250 into a 1-L graduated cylinder ([final] = 0.1%)
- Place 500 ml of methanol into the graduated cylinder ([final] = 50%)
- Place 100 ml of acetic acid into the graduated cylinder ([final] = 10%)
- Place 400 ml of dH2O into the graduated cylinder ([final] = 40%)
- Store in a bottle at room temperature
- Destaining solution
- Place 500 ml of methanol into a 1-L graduated cylinder ([final] = 50%)
- Place 100 ml of acetic acid into the graduated cylinder ([final] = 10%)
- Place 400 ml of dH2O into the graduated cylinder ([final] = 40%)
- Store in a bottle at room temperature
- Place 500 ml of methanol into a 1-L graduated cylinder ([final] = 50%)
- 1x Tris-glycine-SDS buffer
- Place 100 ml of 10x Tris-glycine-SDS buffer into a 1-L graduated cylinder
- Fill with dH2O up to 1 L
- Store in a bottle at room temperature
- Buffers to prepare on Day 4 for purification–Part 1
Note: All the following buffers should be freshly prepared. Keep buffers on ice. Prepare 10 ml of each buffer per cell pellet from 1 L of culture. - Elution buffer (10 ml [Final volume])
- Place 60 mg NaH2PO4 into a 50-ml conical tube ([final] = 50 mM)
- Add 175 mg NaCl ([final] = 300 mM)
- Add 170 mg imidazole ([final] = 250 mM)
- Add 1 ml glycerol ([final] = 10%)
- Add 8 ml dH2O
- Adjust pH to 8.0 with 10 N NaOH
- Bring final volume up to 10 ml with dH2O
- Resuspension buffer (10 ml [Final volume])
- Place 9.2 ml 2x lysis buffer into a 50-ml conical tube
- Add 800 µl elution buffer
- Add 1 cOmplete tablet protease inhibitor (free EDTA)
- Cap tube tightly and vortex until the tablet has completely dissolved (30 sec-1 min)
- Add 10 µl Triton X-100 ([final] = 0.1%)
- Invert tube gently until the Triton X-100 has completely dissolved
- Equilibration buffer (10 ml [Final volume])
- Place 5 ml 2x lysis buffer into a 50-ml conical tube
- Add 5 ml dH2O
- Wash 1 buffer (10 ml [Final volume])
- Place 5 ml 2x lysis buffer into a 50-ml conical tube
- Add 800 µl elution buffer
- Add 4.2 ml dH2O
- Wash 2 buffer ( 10 ml [Final volume])
- Place 5 ml 2x lysis buffer into a 50-ml conical tube
- Add 800 µl elution buffer
- Add 3.2 ml dH2O
- Add 1 ml glycerol ([final] = 10%)
Acknowledgments
A. H-A. is supported by the University of Alabama (UA) College of Arts and Sciences; a grant from the UA College Academy of Research, Scholarship, and Creative Activity (CARSCA); and the National Institutes of Health [5K22AI113106-02]. This protocol was adapted from that published in Hatoum-Aslan et al., J Biol Chem, 2013.
References
- Cao, L., Gao, C. H., Zhu, J., Zhao, L., Wu, Q., Li, M. and Sun, B. (2016). Identification and functional study of type III-A CRISPR-Cas systems in clinical isolates of Staphylococcus aureus. Int J Med Microbiol 306(8): 686-696.
- Christensen, G. D., Baddour, L. M. and Simpson, W. A. (1987). Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun 55(12): 2870-2877.
- Cogen, A. L., Yamasaki, K., Sanchez, K. M., Dorschner, R. A., Lai, Y., MacLeod, D. T., Torpey, J. W., Otto, M., Nizet, V., Kim, J. E. and Gallo, R. L. (2010). Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 130(1): 192-200.
- Conlan, S., Kong, H. H. and Segre, J. A. (2012). Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS One 7(10): e47075.
- Furuya, E. Y. and Lowy, F. D. (2006). Antimicrobial-resistant bacteria in the community setting. Nat Rev Microbiol 4(1): 36-45.
- Goldberg, G. W., Jiang, W., Bikard, D. and Marraffini, L. A. (2014). Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting. Nature 514(7524): 633-637.
- Golding, G. R., Bryden, L., Levett, P. N., McDonald, R. R., Wong, A., Graham, M. R., Tyler, S., Van Domselaar, G., Mabon, P., Kent, H., Butaye, P., Smith, T. C., Kadlec, K., Schwarz, S., Weese, S. J. and Mulvey, M. R. (2012). Whole-genome sequence of livestock-associated st398 methicillin-resistant staphylococcus aureus Isolated from Humans in Canada. J Bacteriol 194(23): 6627-6628.
- Grice, E. A. and Segre, J. A. (2011). The skin microbiome. Nat Rev Microbiol 9(4): 244-253.
- Harris, L. G. and Richards, R. G. (2006). Staphylococci and implant surfaces: a review. Injury 37 Suppl 2: S3-14.
- Hatoum-Aslan, A., Maniv, I., Samai, P. and Marraffini, L. A. (2014). Genetic characterization of antiplasmid immunity through a type III-A CRISPR-Cas system. J Bacteriol 196(2): 310-317.
- Hatoum-Aslan, A., Samai, P., Maniv, I., Jiang, W. and Marraffini, L. A. (2013). A ruler protein in a complex for antiviral defense determines the length of small interfering CRISPR RNAs. J Biol Chem 288(39): 27888-27897.
- Iwase, T., Uehara, Y., Shinji, H., Tajima, A., Seo, H., Takada, K., Agata, T. and Mizunoe, Y. (2010). Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465(7296): 346-349.
- Lai, Y., Cogen, A. L., Radek, K. A., Park, H. J., Macleod, D. T., Leichtle, A., Ryan, A. F., Di Nardo, A. and Gallo, R. L. (2010). Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 130(9): 2211-2221.
- Lowy, F. D. (1998). Staphylococcus aureus infections. N Engl J Med 339(8): 520-532.
- Maniv, I., Jiang, W., Bikard, D. and Marraffini, L. A. (2016). Impact of different target sequences on type III CRISPR-Cas immunity. J Bacteriol 198(6): 941-950.
- Marraffini, L. A. (2015). CRISPR-Cas immunity in prokaryotes. Nature 526(7571): 55-61.
- Marraffini, L. A. and Sontheimer, E. J. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322(5909): 1843-1845.
- Naik, S., Bouladoux, N., Linehan, J. L., Han, S. J., Harrison, O. J., Wilhelm, C., Conlan, S., Himmelfarb, S., Byrd, A. L., Deming, C., Quinones, M., Brenchley, J. M., Kong, H. H., Tussiwand, R., Murphy, K. M., Merad, M., Segre, J. A. and Belkaid, Y. (2015). Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520(7545):104-108.
- Nair, D., Memmi, G., Hernandez, D., Bard, J., Beaume, M., Gill, S., Francois, P., Cheung, A. L. (2011). Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J Bacteriol 193(9): 2332-2335.
- National Nosocomial Infections Surveillance, S. (2004). National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 32(8): 470-485.
- Otto, M. (2009). Staphylococcus epidermidis – the “accidental” pathogen. Nat Rev Microbiol 7(8):555-567.
- Samai, P., Pyenson, N., Jiang, W., Goldberg, G. W., Hatoum-Aslan, A. and Marraffini, L. A. (2015). Co-transcriptional DNA and RNA cleavage during type III CRISPR-Cas immunity. Cell 161(5): 1164-1174.
- Stryjewski, M. E. and Chambers, H. F. (2008). Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46 Suppl 5: S368-377.
- Walker, F. C., Chou-Zheng, L., Dunkle, J. A. and Hatoum-Aslan, A. (2016). Molecular determinants for CRISPR RNA maturation in the Cas10-Csm complex and roles for non-Cas nucleases. Nucleic Acids Res. 45(4): 2112-2123.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chou-Zheng, L. and Hatoum-Aslan, A. (2017). Expression and Purification of the Cas10-Csm Complex from Staphylococci. Bio-protocol 7(11): e2353. DOI: 10.21769/BioProtoc.2353.
Category
Microbiology > Microbial biochemistry > Protein > Isolation and purification
Molecular Biology > Protein > Expression
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link