- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Stereotaxic Surgery for Suprachiasmatic Nucleus Lesions in Mice
Published: Vol 7, Iss 12, Jun 20, 2017 DOI: 10.21769/BioProtoc.2346 Views: 19381
Reviewed by: Xi FengXiaoyu LiuHélène M. Léger

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1645 Views

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2678 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1663 Views
Abstract
Site-specific lesions are invaluable methods for investigating the function of brain regions within the central nervous system and can be used to study neural mechanisms of behaviors. Precise stereotaxic surgery is required to lesion small regions of the brain such as the suprachiasmatic nucleus (SCN), which harbors the master circadian clock. In this protocol, we describe stereotaxic surgery optimized for bilateral lesion of the mouse SCN by loading electric current. Success of the SCN lesion is verified histologically and behaviorally by monitoring arrhythmic locomotor activity. The SCN-lesioned mouse allows for the evaluation of behavioral, biochemical, and physiological consequences of ablation of the master circadian clock.
Keywords: Suprachiasmatic nucleusBackground
The suprachiasmatic nucleus (SCN) is a small region within the hypothalamus of the mammalian brain. It is positioned bilaterally above the optic chiasm and contains approximately 20,000 neurons. The SCN is known as the location of the master circadian oscillator (clock) and is required for synchronization with the light-dark cycle. Ablation of the SCN is a useful strategy for assessing the physiological influence of the master circadian clock.
An electrolytic lesion of the SCN has advantages that enable fast and localized ablation of the master circadian clock in comparison to gene modification by virus injection or SCN-specific promoters. Location of the lesion by electrical impulse can be verified right after surgery by Nissl staining, and monitoring activity rhythm can be started one day after the surgery. In addition, lesion by administration of chemicals often results in non-specific damage and thus it is not as precise as lesion by electrical impulse, especially for small targets such as SCN. Therefore, this protocol provides a useful strategy to evaluate effects (outputs) of master circadian clock.
Materials and Reagents
- Adult mouse (C57BL/6J, usually 8-14 weeks old)
- Ketamine (Daiichi Sankyo Propharma, Ketalar for intramuscular injection 500 mg)
- Xylazine (Bayer, Serakutaru 2% injection)
- Bacteriostatic saline (Otsuka pharmaceutical, 20 ml ampoule)
- 70% ethanol
- For confirmation of SCN-lesioning
- Glacial acetic acid
- 0.2% cresyl violet acetate solution (see Recipes)
- Filter paper
- Glacial acetic acid
Equipment
Note: Refer to Figures 1, 2, 3 for the Equipment used in this protocol.
- Stereotaxic frame (NARISHIGE, model: SR-6M-HT )
- Stereotaxic micromanipulator (NARISHIGE, model: SM-15R/L )
- Wide-field dissecting microscope (ZEISS, model: Stemi 2000 ) with boom stand
- Cold light source (ZEISS, model: CL 1500 ECO )
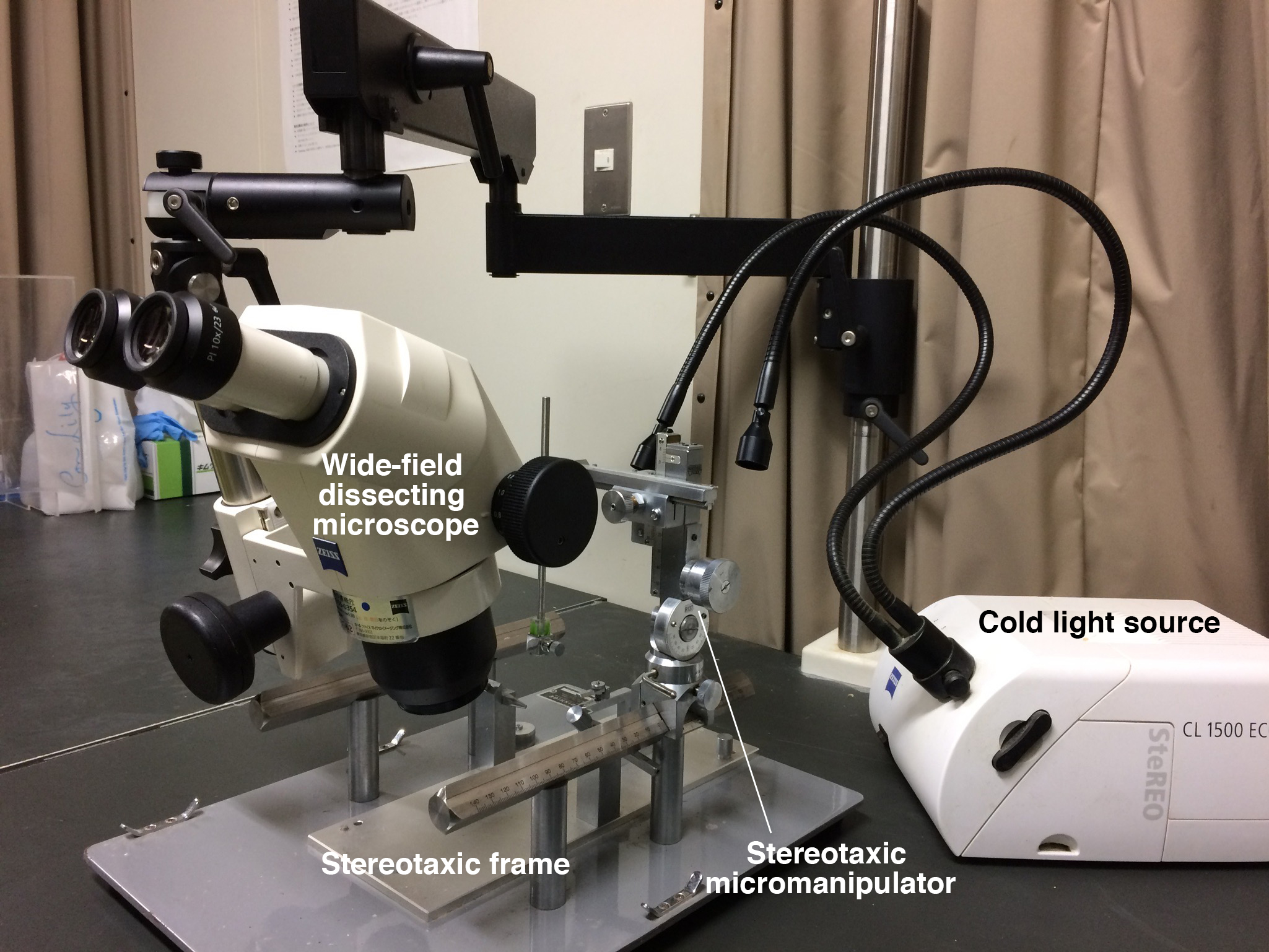
Figure 1. Equipment list 1-4 - Auxiliary ear bar (NARISHIGE, model: EB-5N )
- Scissors (e.g., Fine Science Tools, catalog number: 14068-12 )
- Scalpel (FEATHER Safety Razor, catalog number: No.11 )
- Surgical needle with suture (e.g., NATSUME SEISAKUSHO, catalog number: C21-40 )
- Hemostats (e.g., Fine Science Tools, catalog number: 13008-12 )
- Forceps (e.g., Fine Science Tools, catalog number: 11009-13 or 11008-13 )
- Tuberculin syringe with needle (e.g., Terumo Medical, catalog numbers: SS-01T and NN-2719S )
- Razor (e.g., FEATHER Safety Razor, catalog number: FAS-10 )
- Cotton swabs (e.g., Q-tip)
- Heating pad (e.g., electric heating pad for pets)
- Hand drill with engraving cutter (DREMEL, model: 106 )
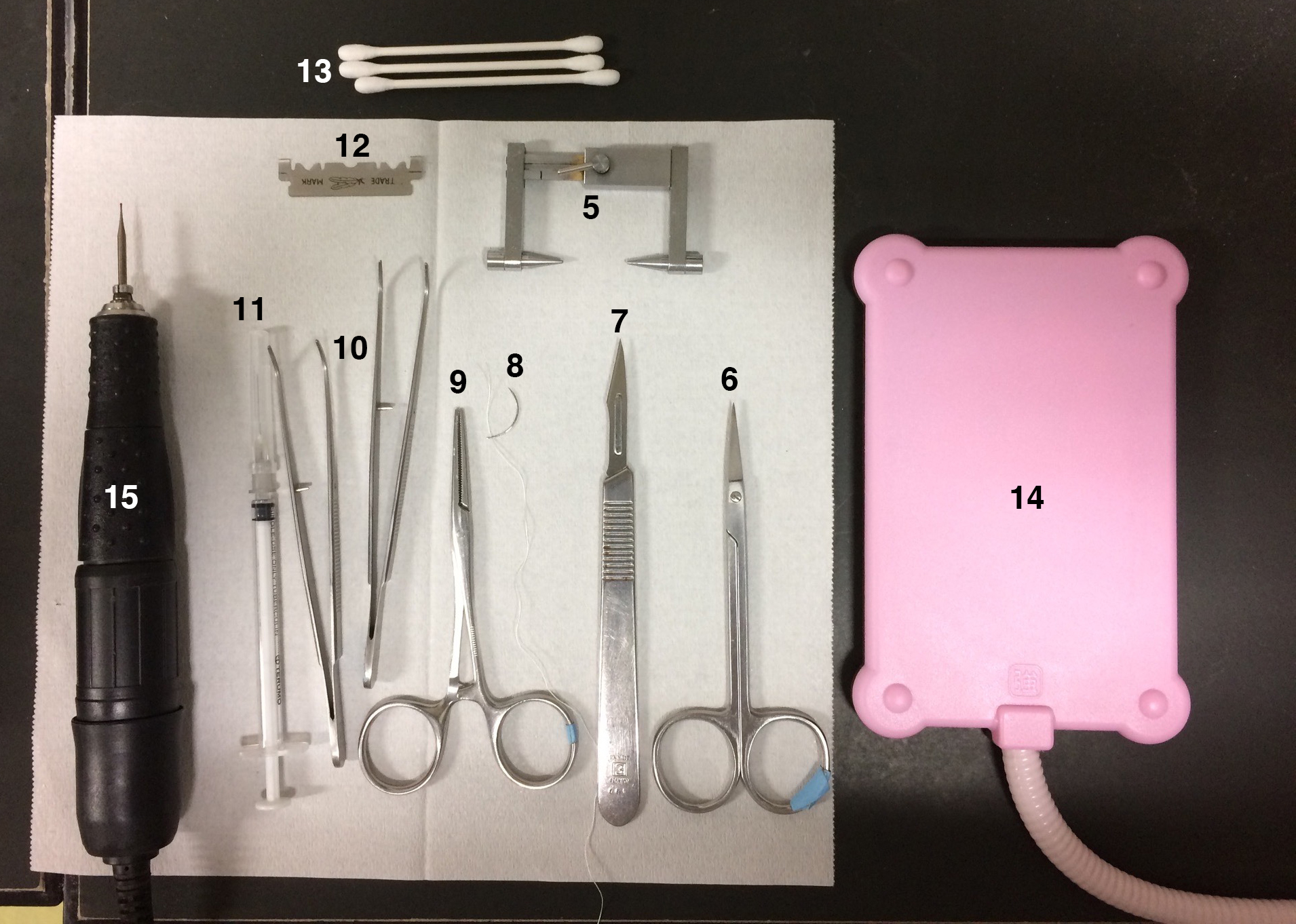
Figure 2. Equipment list 5-15 - Lesion-making device (Ugo Basile, catalog number: 53500 )
- Electrode (100 μm, coated with epoxy except for 200 μm at the tip, Neuroscience)
- Plug connecter cables with crocodile clip
- Area sensor with an infrared detector for confirmation of SCN-lesioning (EK, catalog number: PS-3241 )
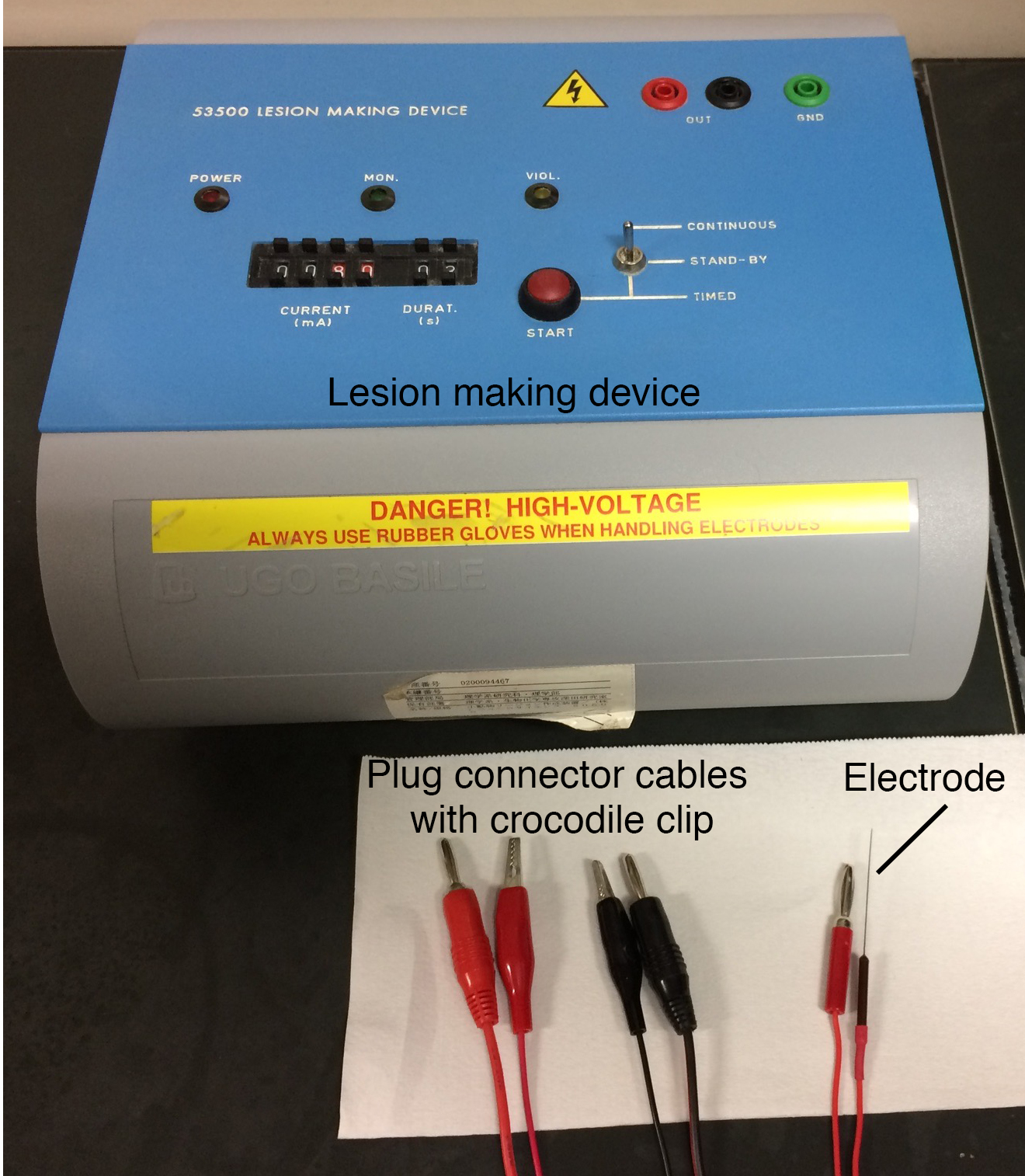
Figure 3. Equipment list 16-18
Software
- ClockLab software
Procedure
This protocol is based on structural features on the skull surface (bregma and lambda) as reference points for determining the target position (Figure 5). Bregma and lambda are defined as the intersections of the sagittal suture with the coronal and lambdoid sutures, respectively. A mouse brain atlas (e.g., Paxinos and Franklin, 2001) allows the determination of the anterior-posterior, medial-lateral, and dorsal-ventral coordinates of the brain structures from measurement of these skull features.
- Anesthetize mouse
- Anesthetize the mouse with an intraperitoneal injection (20 µl/g of body weight) of a mixture of ketamine (7 mg/ml) and xylazine (0.44 mg/ml) dissolved in bacteriostatic saline. The animal should reach surgical anesthesia within 5-10 min. Shave the fur of the top of the skull after the mouse has reached full anesthesia.
Note: Depth of anesthesia is assessed by the corneal blink and tail-pinch reflexes. Wait until the reflexes are disappeared, if the mouse is not well anesthetized. Anesthesia should persist for approximately 1 h. - Mount mouse to a stereotaxic frame (Video 1)Video 1. Procedure of setting mouse to a stereotaxic frame. This video describes how to mount a mouse to a stereotaxic frame. The videoclip begins with insertion the auxiliary ear bar into the external auditory meatus.
- Before mounting the mouse to the stereotaxic frame, insert the auxiliary ear bar. Guide the tips of the auxiliary ear bar into the external auditory meatus and tighten the screw.
Note: Small popping sounds are heard when the tips are properly inserted to the tympanic membranes. Make sure that the auxiliary ear bar and the midline of the mouse are vertically placed. - Insert the ear bars of the stereotaxic frame into the auxiliary ear bar. Lock the ear bars tightly. When done correctly, the animal’s head should be centered by symmetrical adjustment of the ear bars.
- Slide the incisor bar into the mouth and place incisors into the hole. Apply downward pressure to the nose while sliding the incisor bar back until it stops. Tighten the nose clamp and incisor bar firmly (Figure 4).

Figure 4. A mouse mounted in the stereotaxic frame - Surgery (Video2)Video 2. Stereotaxic surgery. The video shows steps for the surgery.
Note: To maintain body temperature, it is recommended to place the mouse on a heating pad (38 °C) during surgery. - Disinfect the scalp with 70% ethanol and make an incision along the midline with a scalpel. Expose the skull and scrape away the pericranial tissues with a cotton swab.
- Fit the stereotaxic micromanipulator to the stereotaxic frame and attach the electrode.
- Perform dorsoventral measurements at bregma and lambda by lowering the tip of the electrode until it just touches the skull. Then, adjust the incisor bar to bring both bregma and lambda lines at the same horizontal level (Figure 5).
Note: At this point, make sure that both of the bregma and the lambda are on the midline. If these are not on the midline, it is necessary to remount the mouse on the stereotaxic frame.
Figure 5. Dorsal surface of the mouse skull. Bregma and lambda are defined as the points of intersections of the sagittal suture with the coronal and lambdoid sutures, respectively. - Using a dissecting microscope, set zero at bregma. Set the electrode at the target coordinates: 0.2 mm caudal to bregma and 0.23 mm bilateral to the midline for an adult C57BL/6J mouse. Mark the targets with the pencil on the skull.
- Drill two small burr holes through the skull at the marked targets for the bilateral targets. Lower the electrode into the hole 5.9 mm below the skull surface for an adult C57BL/6J mouse. If bleeding is observed after drilling, stop bleeding by applying pressure to the whole by a cotton swab.
Note: The correct target coordinates will vary depending on the age or strain of the mouse. Pilot surgeries may be necessary to refine the target coordinates. - Setting lesion making device
- Connect the electrode to the binding post (+). Pinch the nose clamp with one of the clips of the plug connector cable and connect it to the binding post (-). Pinch the mouse tail with the other clip of the plug connector cables and connect to the binding post (G) (Figure 6).
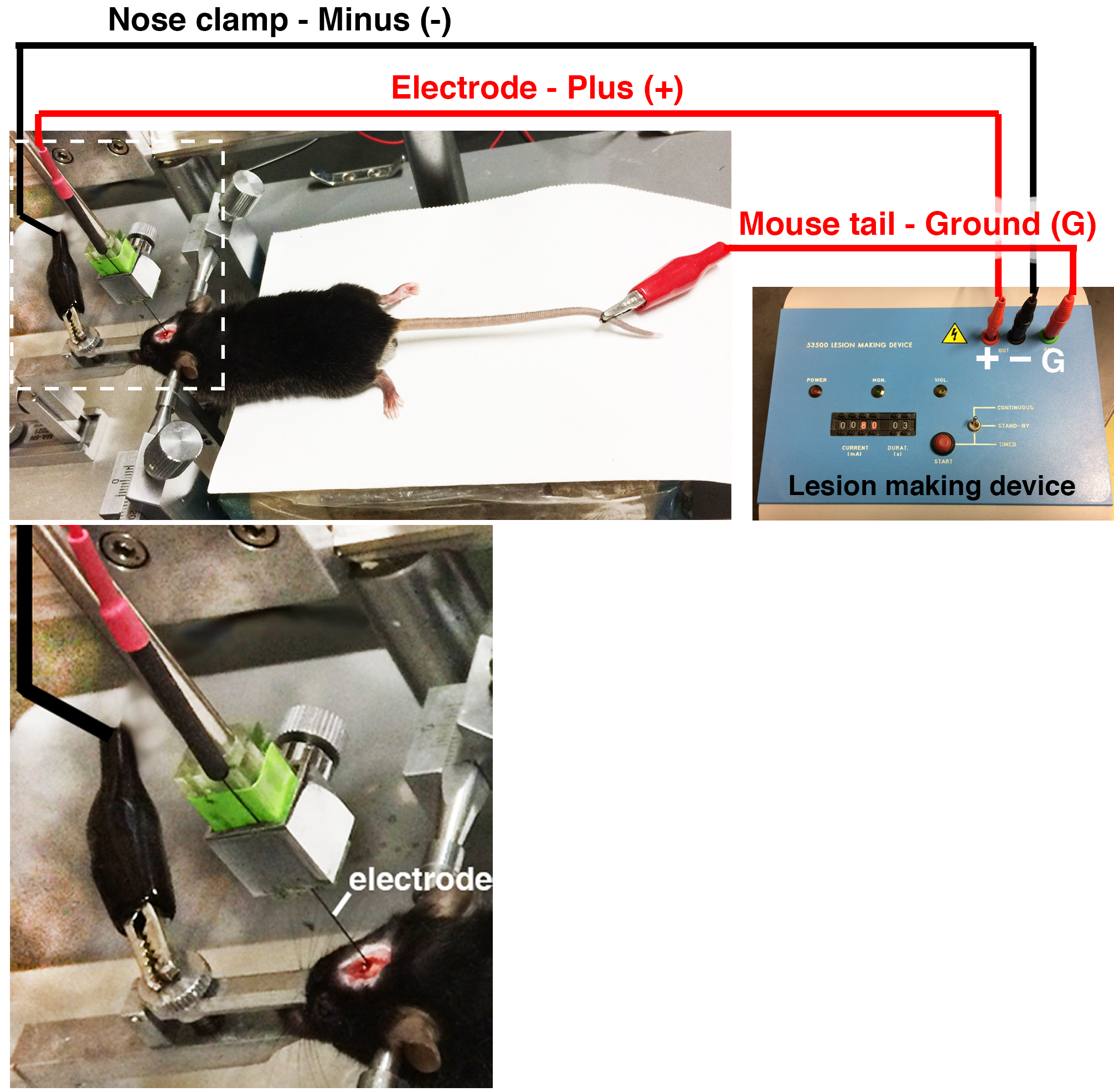
Figure 6. The connection of the lesion-making device to the mouse
- Connect the electrode to the binding post (+). Pinch the nose clamp with one of the clips of the plug connector cable and connect it to the binding post (-). Pinch the mouse tail with the other clip of the plug connector cables and connect to the binding post (G) (Figure 6).
- SCN-lesion
- Deliver an electric current of 0.8 mA for 3 sec using the lesion-making device. For the sham operation, use the same surgical procedure but do not deliver a current.
- Remove the electrode and suture the incision using surgical needle and thread (Video 3). Remove the mouse from the stereotaxic frame and auxiliary ear bars.
- Keep warm the mouse on a heating pad (38 °C) during recovery (approx. 30 min).
Video 3. Suture of incision. This video provides a demonstration of suture of the incision in the end of surgery.
- Deliver an electric current of 0.8 mA for 3 sec using the lesion-making device. For the sham operation, use the same surgical procedure but do not deliver a current.
- Confirmation of SCN-lesion
To confirm arrhythmicity induced by the SCN-lesion, monitor the rhythm of free-moving activity in constant darkness using an area sensor with an infrared detector (Figure 7). The activity rhythm can be analyzed using ClockLab software. The SCN lesion can also be confirmed by Nissl staining of the SCN slices using cresyl violet solution (Figure 8).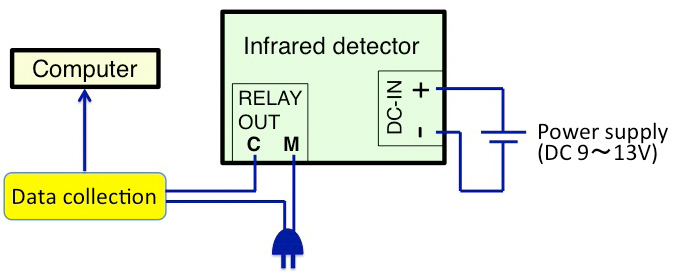
Figure 7. The schematic chart of the infrared detector setup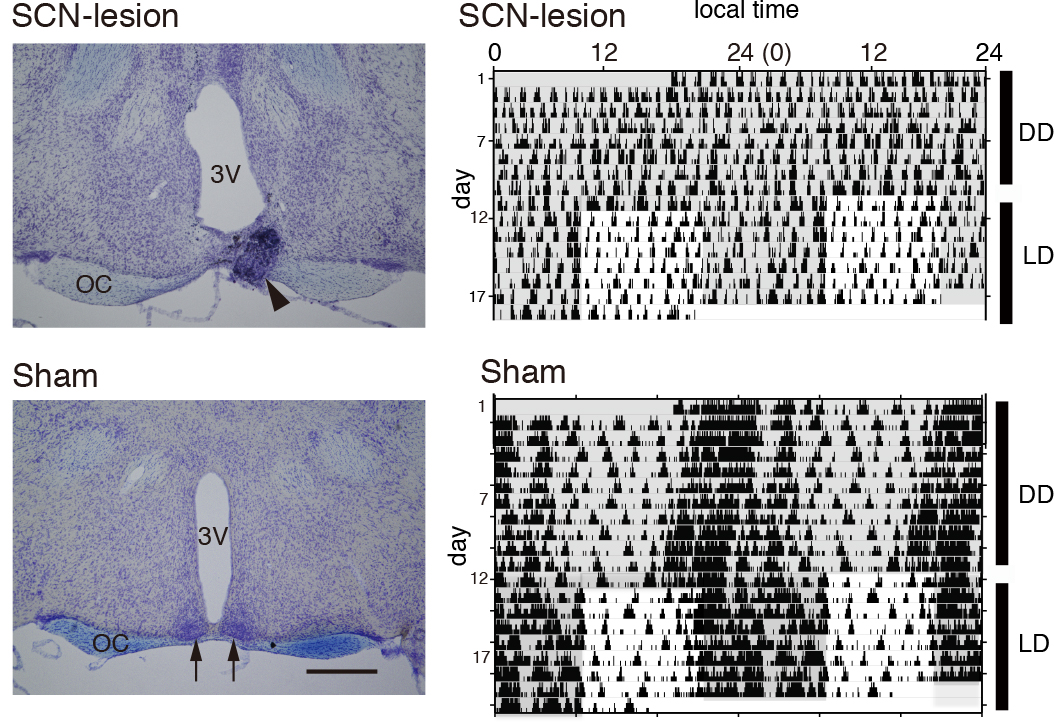
Figure 8. Confirmation of the SCN-lesion. Shown are the representative Nissl-stained coronal brain sections of a mouse 1 month after SCN-lesion (left panels) and the double-plotted actograms (right panels) of the SCN-lesioned mouse (upper panels) and sham-operated one (lower panels). In the Nissl-staining (left panels), the bilateral SCN is lost in the section of SCN-lesioned mouse (upper panel), while the SCN (arrows) in the section of sham-operated mouse is intact (lower panel). Electrolytic lesioning frequently results in formation of tissue debris (arrowhead). 3 V, third ventricle. OC, optic chiasm. Scale bar = 500 µm. When the activity is monitored under constant dark (DD) condition, the SCN-lesioned mouse shows arrhythmic locomotor behavior (upper panel), whilst the sham-operated mouse shows a normal circadian rhythm. Shaded areas on the actograms signify the dark period. (Data are cited from Shimizu et al., 2016).
Data analysis
Analysis for activity rhythm: ClockLab software collects and analyzes the data automatically. Chi-square periodgram analysis is used to assess rhythmicity. If the SCN lesion surgery is successful, no periodogram peaks (as defined with a statistical significance level P < 0.05) will be detected in a 24 h period.
Notes
- The entire surgical protocol takes approximately 30 min per animal.
- The most important step to ensure accuracy is to mount the head of the mouse correctly on the stereotaxic frame.
Recipes
- 0.2% cresyl violet acetate solution
- Dissolve 2.0 g cresyl violet in 990 ml distilled water
- Add 10 ml glacial acetic acid
- Make up to 1,000 ml with distilled water and agitate it for 1 h
- Filtrate the solution through a filter paper
Acknowledgments
This work is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Paxinos, G. and Franklin, K. B. J. (2001). The Mouse Brain in Stereotaxic Coordinates, Deluxe Edition of the Atlas. Academic Press.
- Shimizu, K., Kobayashi, Y., Nakatsuji, E., Yamazaki, M., Shimba, S., Sakimura, K. and Fukada,Y. (2016). SCOP/PHLPP1β mediates circadian regulation of long-term recognition memory. Nat Commun 7: 12926.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Shimizu, K. and Fukada, Y. (2017). Stereotaxic Surgery for Suprachiasmatic Nucleus Lesions in Mice. Bio-protocol 7(12): e2346. DOI: 10.21769/BioProtoc.2346.
Category
Neuroscience > Neuroanatomy and circuitry > Animal model
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










