- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Whole Mammary Gland Transplantation in Mice Protocol
Published: Vol 7, Iss 11, Jun 5, 2017 DOI: 10.21769/BioProtoc.2326 Views: 8982
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Establishment of Patient-Derived Xenografts in Mice
Dongkyoo Park [...] Xingming Deng
Nov 20, 2016 15570 Views

A Murine Orthotopic Allograft to Model Prostate Cancer Growth and Metastasis
Robert M. Hughes [...] Paula J. Hurley
Feb 20, 2017 12188 Views

Qualitative in vivo Bioluminescence Imaging
Devbarna Sinha [...] Pritinder Kaur
Sep 20, 2018 10950 Views
Abstract
Whole Mammary Gland Transplantation involves transplanting an excised mammary gland into another, more suitable host. This method can be used to extend the life of a mammary gland past the mouse’s life span by transplanting the mammary gland of an older mouse into a young healthy mouse. As you can see in the video below (Video 1), by attaching it to the abdomen of the mouse, the gland will receive a steady blood supply and both epithelial and stromal cells will remain viable for up to one year. Although this method is not used often, it has been part of several experiments including determining whether the stroma or epithelium is the primary target in chemically induced mouse mammary tumorigenesis (Medina and Kittrell, 2005). To monitor transplants, palpate every week for tumor formation. The transplanted mammary gland may also be passaged serially every 8-10 weeks. Keep transplanted gland in the same mouse for no longer than one year.
Materials and Reagents
- Surgical gloves (Cardinal Health, catalog number: 2D72N80X )
- 1 ml Tuberculin (TB) syringe (BD, catalog number: 309625 )
- Dissolvable suture, size: 0000
- Animals: 8-12-week old female mice
Note: Excised gland will need to be transplanted into the same strain of mouse. If donor and recipient are of different strains, the recipient must be immunocompromised (i.e., gland excised from Balb/c mouse and transplanted to NSG mouse). - Nembutal sodium solution CII (Pentobarbital sodium injection, USP) (Akorn, Oak Pharmaceuticals, NDC 76478-501-20 )
- Ketofen® (Ketoprofen) (Zoetis, catalog number: 10004029 )
- 70% ethanol
Equipment
- Balance
- Hair clipper (Remington, model: PG525 )
- Hemostatic forceps (Roboz Surgical Instruments, catalog number: RS-7131L )
- Sharp/Ball Tip scissors (Fine Science Tool, catalog number: 14086-09 )
- Rat tooth forceps (Sklar Surgical Instruments, catalog number: 19-1260 )
- Curved forceps (Fine Science Tool, catalog number: 11052-10 )
- Heating pad (Sunbeam Products, catalog number: 000771-810-000U )
- Wound Clip applier, remover and clips (BD, catalog number: 427638 )
Procedure
- Mammary gland excision (not included in video)
- Using the same preparation for surgery and steps C1-C2 of the surgery procedure described below, locate the mammary gland to be excised and using curved forceps, make sure the whole gland is exposed and completely separated from the body cavity.
- Use rat tooth forceps to grasp the gland and using sharp scissors begin cutting the gland away from the skin until the whole gland is free from the skin and in one piece.
- Immediately transfer the excised gland to the recipient mouse and continue with step C3 of the surgery procedure.
- Using the same preparation for surgery and steps C1-C2 of the surgery procedure described below, locate the mammary gland to be excised and using curved forceps, make sure the whole gland is exposed and completely separated from the body cavity.
- Prepare the mice for surgery
- Weigh mouse.
- Using 1 ml TB syringe, administer 40 mg/g body weight of Nembutal intraperitoneally to one side of the abdomen (this will provide 1 h of sedation).
- Wait until the mouse has fallen asleep; confirm by checking hind limb reflex by pinching foot.
- Use clippers to shave the abdomen.
- Using 1 ml TB syringe, administer 5 mg/kg body weight Ketofen® intraperitoneally on the opposing side of ketamine injection site. This is for pain relief.
- Place the mouse on a surgical board and secure limbs with surgical tape.
- Sterilize the abdomen with 70% alcohol.
- Weigh mouse.
- Surgery
- Using the ball tip scissors, make an inverted Y incision to expose the abdominal wall and inguinal fat pads. Begin on the midline slightly below inguinal nipple and extended up about 1 inch; extending the other two cuts between the fourth and fifth nipples on either side.
- Hold the skin with rat tooth forceps and using the curved forceps, separate the skin from the abdominal wall. Secure skin flaps with pins to expose abdominal wall.
- Place excised gland on the abdominal wall, on either side of the midline. Make sure gland is lying flat against the wall and is not twisted as seen below (Figure 1).
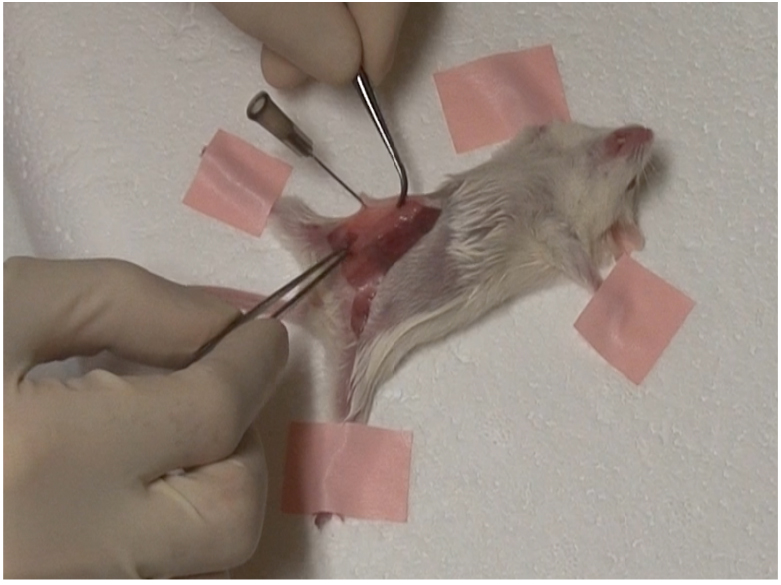
Figure 1. Excised gland must lie flat against abdominal wall - Using hemostatic forceps, curved forceps and suture, suture each end of the transplanted gland to abdominal wall as seen below (Figure 2).
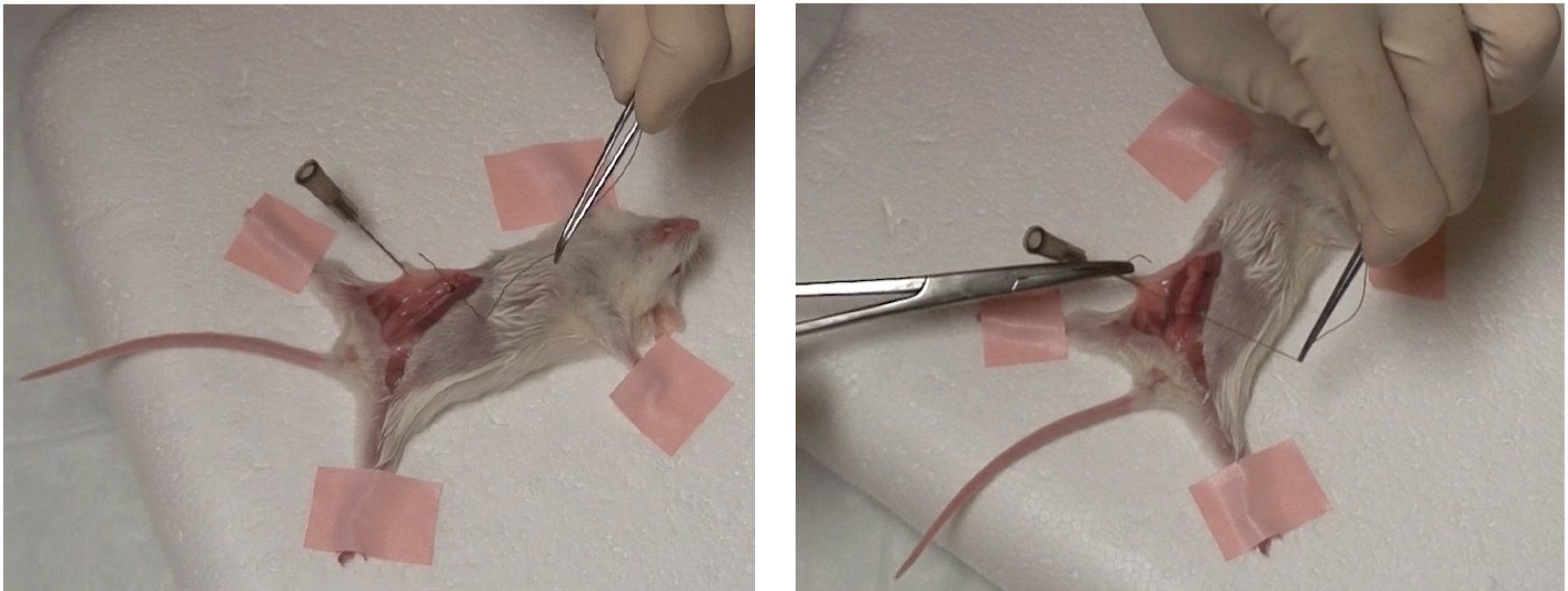
Figure 2. One suture in place at both ends of excised gland - Reposition the skin flaps and close the incision with wound clips.
- Using the ball tip scissors, make an inverted Y incision to expose the abdominal wall and inguinal fat pads. Begin on the midline slightly below inguinal nipple and extended up about 1 inch; extending the other two cuts between the fourth and fifth nipples on either side.
- Post-surgery
- Allow mouse to recover on heating pad set to lowest setting (~110 F). Allow time for the mouse to regain consciousness before returning to the cage (30 min-1 h).
Note: Keep in mind that the sedation lasts 1 h. - Administer 5 mg/kg body weight of Ketofen® intraperitoneally on either side of the abdomen 24 h later.
- Remove wound clips 7-10 days post-surgery.
- To monitor, palpate weekly for tumor formation.
- Allow mouse to recover on heating pad set to lowest setting (~110 F). Allow time for the mouse to regain consciousness before returning to the cage (30 min-1 h).
Notes
Recipients were 8- to 12-week-old virgin female NSG, which were purchased from Jackson Laboratories. Animal experiments were conducted following protocols approved by the University of Kansas School of Medicine Animal Care and Use and Human Subjects Committee. All researchers must have this protocol approved by their own institution before performing the procedure.
Acknowledgments
This protocol was developed in the Department of Molecular and Cellular Biology, Baylor College of Medicine and Department of Pathology and Laboratory Medicine, University of Kansas Medical School. The work was supported by NCI (1K99/R00 CA127462) to FB.
References
- Medina, D. and Kittrell, F. (2005). Stroma is not a major target in DMBA-mediated tumorigenesis of mouse mammary preneoplasia. J Cell Sci 118(Pt 1): 123-127.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hansford, H., Hong, Y., Kittrell, F., Medina, D. and Behbod, F. (2017). Whole Mammary Gland Transplantation in Mice Protocol. Bio-protocol 7(11): e2326. DOI: 10.21769/BioProtoc.2326.
Category
Cancer Biology > Invasion & metastasis > Animal models > Cell invasion
Cell Biology > Tissue analysis > Tissue isolation
Cell Biology > Cell Transplantation > Allogenic Transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link