- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pituitary Isograft Transplantation in Mice
Published: Vol 7, Iss 11, Jun 5, 2017 DOI: 10.21769/BioProtoc.2317 Views: 9082
Reviewed by: Thirupugal GovindarajanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assessing the Presence of Hematopoietic Stem and Progenitor Cells in Mouse Spleen
Isabelle J. Marié [...] David E. Levy
Jun 5, 2022 3775 Views

Generation and Maintenance of Patient-Derived Endometrial Cancer Organoids
Mali Barbi [...] Semir Beyaz
Oct 20, 2024 2061 Views

Temporally and Spatially Controlled Age-Related Prostate Cancer Model in Mice
Sen Liu [...] Qiuyang Zhang
Jan 5, 2025 2701 Views
Abstract
The mouse pituitary isograft is a technique developed to administer persistent hormone stimulation, thereby increasing cellular proliferation in the mammary tissue (Christov et al., 1993). The pituitary isograft procedure was first described in ‘Induction of Mammary Cancer in Mice without the Mammary Tumor Agent by Isografts of Hypophyses’ by O. Mühlbock and L. M. Boot in 1959 (Muhlbock and Boot, 1959). Since then, the procedure has seen wide use. A pituitary gland is harvested posthumously from a donor mouse and implanted under the renal capsule of the recipient mouse through a small abdominal incision just below the last rib. Once the pituitary gland is implanted, it begins releasing hormones. These secretions increase serum levels of multiple hormones including prolactin, progesterone and 17β-estradiol (Christov et al., 1993). Although the effects of these hormones on cancer cell proliferation, growth, differentiation, and longevity are not well characterized, and, in some cases, controversial, the net effect of a pituitary isograft is to increase the proliferation of murine breast tissue depending upon strain specific characteristics (Lydon et al., 1999).
Below is a protocol describing how to perform the pituitary isograft procedure. After many of the steps, a time reference is listed in parentheses. Each reference corresponds to a time point in the embedded video of the procedure. (Video 1)
Keywords: Pituitary isograft
Background
Because of the simplicity, longevity, and applicability of the pituitary isograft procedure it has seen substantial and varied usage in the decades since its inception. Uniform, long term hormone stimulation in vivo is difficult to achieve; pituitary isografts deliver this capability. Once implanted pituitary isografts remain effective and safe for the duration of the experimental lifespan.
Materials and Reagents
- Surgical gloves (Cardinal Health, catalog number: 2D72N80X )
- 1 ml Tuberculin (TB) syringe (BD, catalog number: 309625 )
- 18 gauge 1 ½ inch trocar (BD, catalog number: 305196 )
- Silk suture 4-0 (Patterson Veterinary Supply, catalog number: 07-891-0230 )
- Wound clips (BD, catalog number: 427631 )
- Sterile cotton swab (Puritan Medical, catalog number: 25803 2WC )
- 8-20-week-old donor and recipient mice of the same strain
- Nembutal sodium solution (Pentobarbital sodium injection, USP) (Akorn, catalog number: NDC 76478-501-20 )
- Ketoprofen (Patterson Veterinary Supply, catalog number: 07-803-7377 )
- Phosphate-buffered saline (PBS) (pH 7.4, 1x, sterile) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- 70% ethanol
- Antibiotic solution
Equipment
- Balance
- Sharp-blunt scissors (Fine Science Tool, catalog number: 14028-10 )
- Straight flat broad tip forceps (Fisher Scientific, catalog number: 16-100-114 )
- Graefe forceps curved serrated (Fine Science Tool, catalog number: 11051-10 )
- Graefe forceps straight 1 x 2 teeth (rat tooth) (Fine Science Tool, catalog number: 11053-10 )
- Animal clippers (Sunbeam Products, Oster, catalog number: 078005010002 )
- Clip applier (BD, catalog number: 427630 )
- Clip remover (BD, catalog number: 427637 )
Procedure
- Donor mouse surgery: Harvesting the pituitary
- Weigh the 8-20-week-old Donor mouse. Donor can be either sex.
- Administer 100 mg/kg Nembutal intraperitoneally, a chemical overdose, eventually stopping the mouse’s respiration. Wait until the mouse has no detectable heartbeat then decapitate using sharp-blunt scissors.
- Peel the skin off the posterior skull starting from the base of the neck moving anteriorly toward the nose, removing connective tissue as needed. (0:30)
- Carefully place the sharp tip of the scissors into the skull of the mouse and cut the skull along the sagittal sutures on either side. (0:40)
- Cut along the anterior-most suture line connecting your previous two incisions. Then using the straight flat broad tip forceps remove the dome of the skull. (0:48)
- Using the straight flat broad tip forceps carefully remove the brain by grasping the parietal lobes and lifting the brain away from the skull. (0:57)
- Locate the pituitary gland between the optic nerves and using the curved Graefe forceps outline the pituitary gland to break the membrane holding it in place. Gently lift the pituitary by cradling it between the points of the forceps. (1:04) (see Figure 1)
- Place donor pituitary gland into room temperature PBS. (1:25)
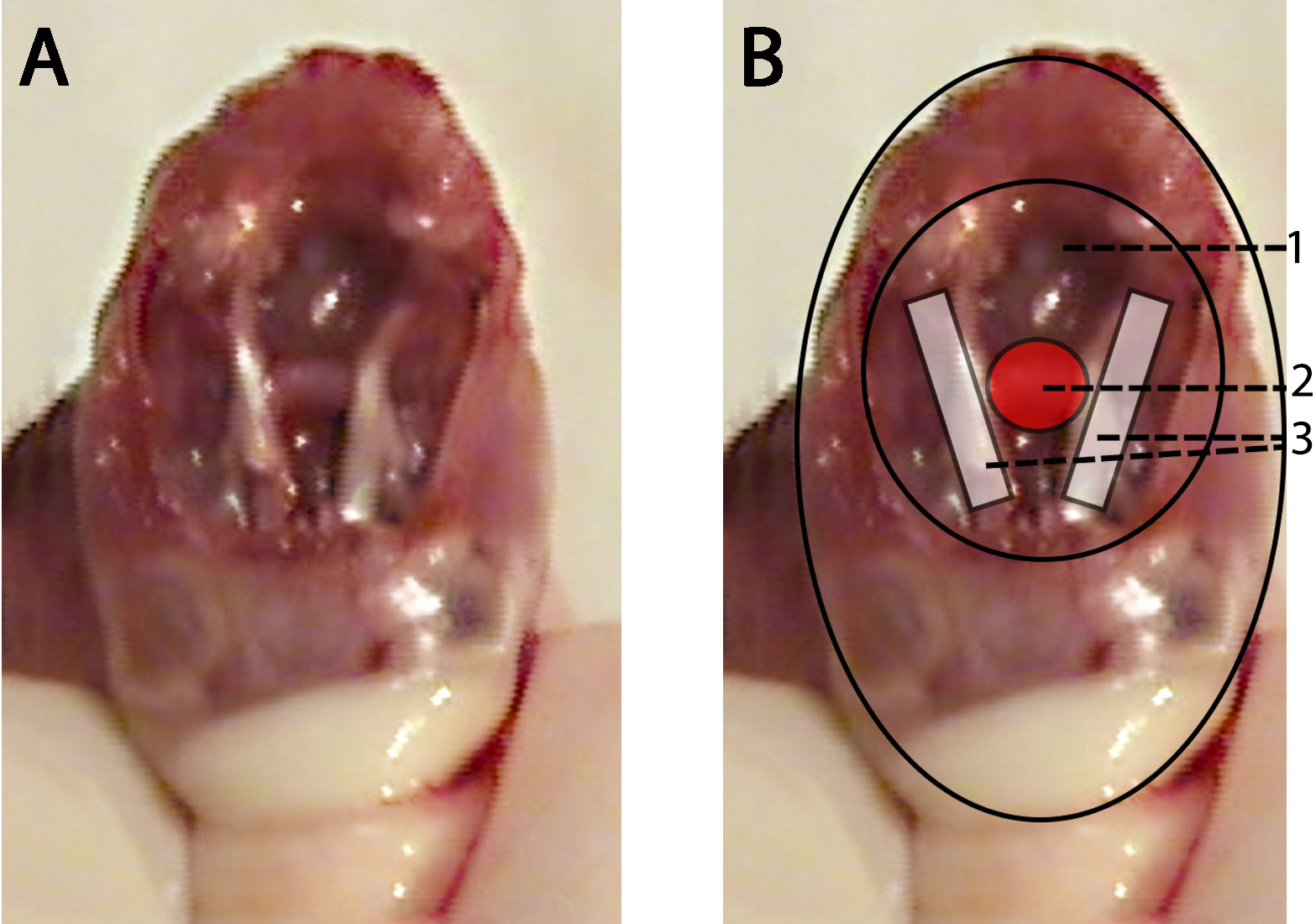
Figure 1. Donor mouse skull with schematic representation showing pituitary gland location. A. Donor mouse skull after brain is removed and the pituitary gland is exposed. B. Donor mouse skull after the brain has been removed with schematic representation overlaid with highlighted regions; base of the cranial cavity (1), Donor mouse pituitary gland (red) (2), donor mouse optic nerves (white) (3).
- Weigh the 8-20-week-old Donor mouse. Donor can be either sex.
- Recipient mouse surgery: Pituitary isograft
- Weigh the 8-20-week-old mouse.
- Using 1 ml Tuberculin syringe, administer 50 mg/kg Nembutal as well as 5 mg/kg ketoprofen intraperitoneally. These dosages will provide 1 h of sedation and twenty-four hours of analgesic respectively.
- Confirm mouse sedation using a toe pinch to a hind limb. If a reflex is observed wait one minute and attempt again. If after ten minutes a reflex is still observed intraperitoneally administer 12.5 mg/kg Nembutal.
- Use clippers to shave the right flank of the mouse from mid ribcage to hip.
- Sterilize the abdomen with 70% alcohol. (1:53)
- Locate the bottom of the rib cage. Using rat tooth forceps, grasp the skin and lift. Using the pointed end of the sharp-blunt scissors, pierce the tented skin and cut to create a one centimeter incision ending at the bottom most rib. (2:03)
- Using rat tooth forceps, grasp the muscles of the abdominal wall exposed by the previous incision and create a smaller incision through the abdominal wall with scissors. (2:17)
- Using the eighteen gauge 1.5-inch long beveled barrel trocar draw some PBS into the barrel to moisten. (2:40)
- Lay the excised pituitary gland onto the beveled edge of the trocar and draw the pituitary gland entirely into the barrel of the trocar. Place carefully aside, making sure to not dislodge the pituitary gland. (2:47)
- Using a sterile cotton swab moistened with PBS, apply pressure ventral to the abdominal incision to expose the kidney through the abdominal wall. Using your thumb and the cotton swab, apply light pressure to keep kidney exposed. (3:25) (see Figure 2)
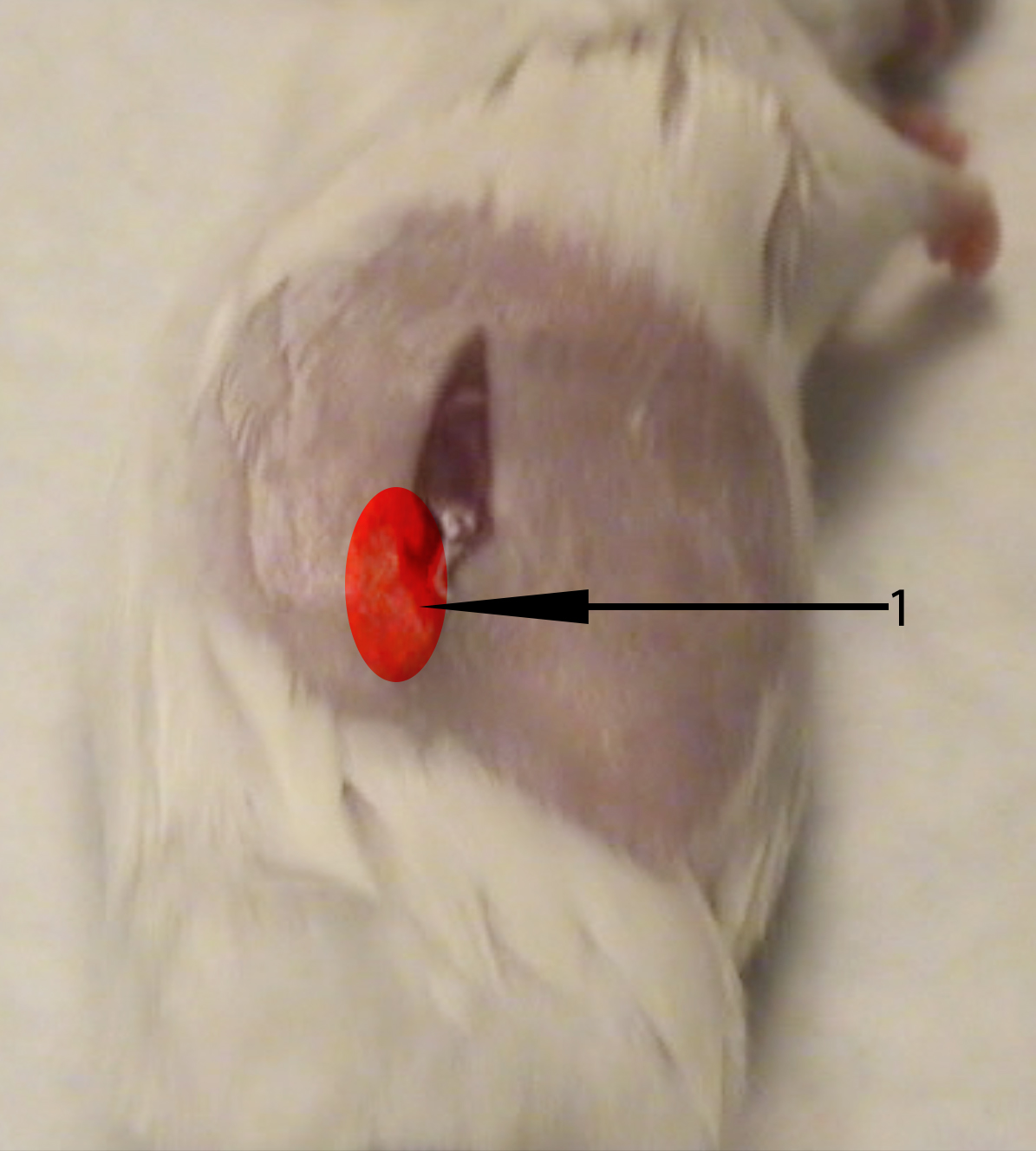
Figure 2. Image of recipient mouse right flank. The recipient mouse’s right kidney can be exposed with a cotton swab and finger by using a light pinching pressure applied deep to the ventral and dorsal sides of the highlighted region (1). - While keeping the kidney exposed, use the sharp tip of the curved Graefe forceps to make a 1 mm hole in the renal capsule. Be sure to keep the forceps parallel to the tabletop to avoid damaging the kidney. (3:35)
- Insert the trocar bevel edge up through the hole in the renal capsule; again, be careful to keep the trocar parallel to the tabletop to avoid damaging the kidney or tearing the renal capsule. Gently inject the pituitary gland into the capsule. The pituitary should be visible through the capsule. (3:50) (see Figure 3)
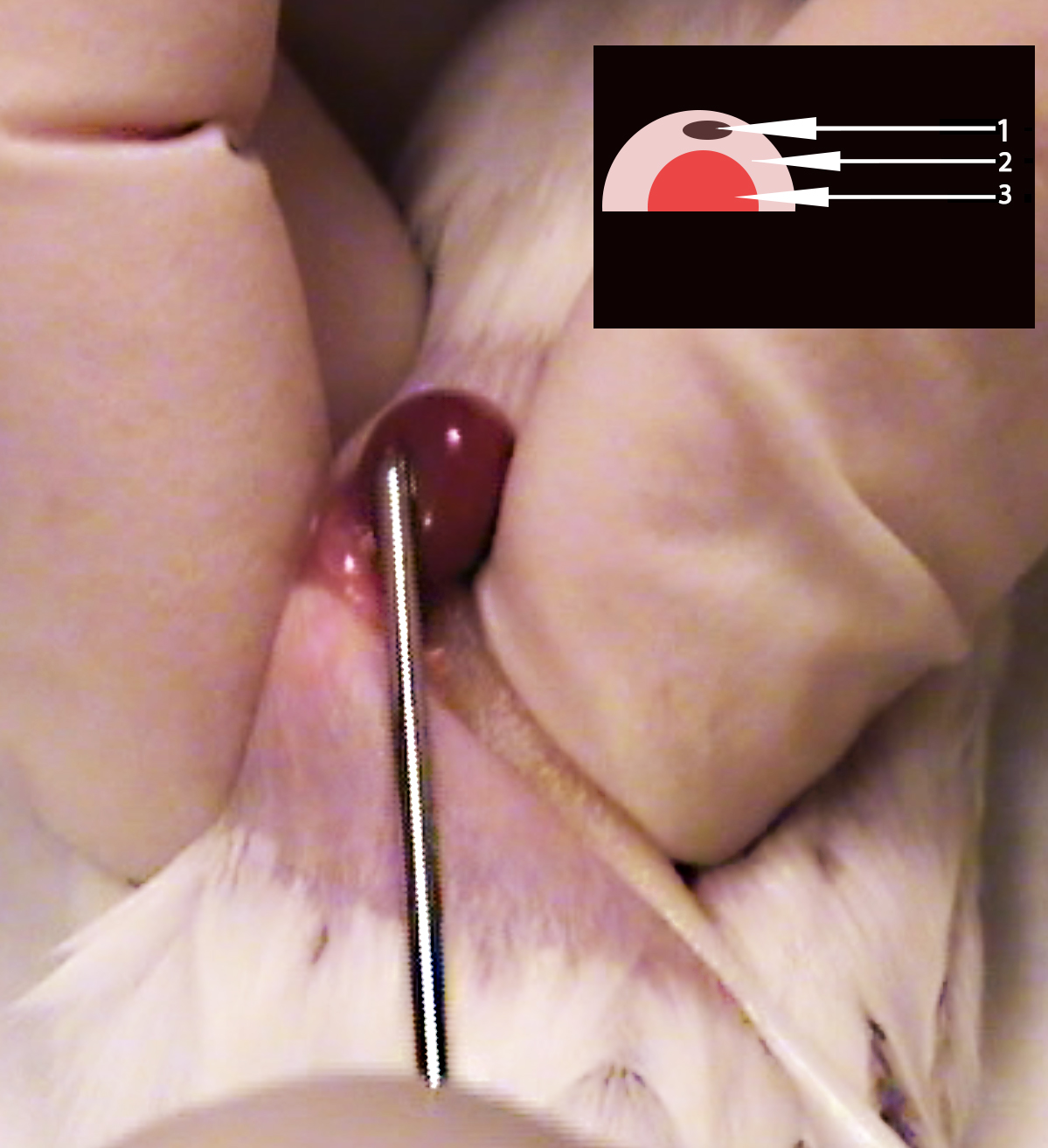
Figure 3. Image depicting correct trocar placement during pituitary gland injection into renal capsule. Notice trocar is parallel with recipient mouse flank and visible through renal capsule. Inset: Schematic representation of proper injection of pituitary gland (1), into exposed renal capsule (2), above the recipient mouse kidney (3). - Using rat tooth forceps lift skin ventral to the incision to place the kidney back into the abdomen. (4:22)
- Place three drops of antibiotic solution directly onto the incision site. (4:31)
- Sew the abdominal wall incision with two simple interrupted sutures. (4:51)
- Close the exterior skin incision with two wound clips. (5:46)
- Weigh the 8-20-week-old mouse.
- Post-surgery
- Place the mouse onto a warm surface. Wait until the righting reflex is restored and the mouse can move about normally. Place mouse back into cage, monitor using regular postoperative criteria.
- Intraperitoneally inject ketoprofen 5 mg/kg 24 h later.
- Remove wound clips after 7-10 days.
- Place the mouse onto a warm surface. Wait until the righting reflex is restored and the mouse can move about normally. Place mouse back into cage, monitor using regular postoperative criteria.
Acknowledgments
This protocol was developed in the Department of Molecular and Cellular Biology, Baylor College of Medicine and Department of Pathology and Laboratory Medicine, University of Kansas Medical School. We thank David Edwards for assistance in the production of the procedure video.
References
- Christov, K., Swanson, S. M., Guzman, R. C., Thordarson, G., Jin, E., Talamantes, F. and Nandi, S. (1993). Kinetics of mammary epithelial cell proliferation in pituitary isografted BALB/c mice. Carcinogenesis 14(10): 2019-2025.
- Lydon, J. P., Ge, G., Kittrell, F. S., Medina, D. and O'Malley, B. W. (1999). Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59(17): 4276-4284.
- Muhlbock, O. and Boot, L. M. (1959). Induction of mammary cancer in mice without the mammary tumor agent by isografts of hypophyses. Cancer Res 19(4): 402-412.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Walker, C., Hong, Y., Kittrell, F., Medina, D., Edwards, D. and Behbod, F. (2017). Pituitary Isograft Transplantation in Mice. Bio-protocol 7(11): e2317. DOI: 10.21769/BioProtoc.2317.
Category
Cancer Biology > General technique > Animal models
Cell Biology > Cell Transplantation > Isograft transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link