- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
ELISPOT Assay to Measure Peptide-specific IFN-γ Production
Published: Vol 7, Iss 11, Jun 5, 2017 DOI: 10.21769/BioProtoc.2302 Views: 20509
Reviewed by: Ivan ZanoniPer AndersonAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Muscle Biopsy Sample Preparation and Proteomics Analysis Based on UHPLC-MS/MS
Jiawei Du [...] Yafeng Song
Dec 20, 2024 1918 Views

Protocol for Screening Host-Targeting Antivirals (HTAs) Using Human PBMCs and pDCs
Zhao Xuan Low [...] Pouya Hassandarvish
Mar 5, 2025 3071 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2359 Views
Abstract
Interferon-gamma (IFN-γ) is crucial for immunity against intracellular pathogens and for tumor control. It is produced predominantly by natural killer (NK) and natural killer T cells (NKT) as well as by antigen-specific Th1 CD4+ and CD8+ effector T cells. When investigating immune responses against pathogens and cancer cells, measuring antigen-specific cytokine-responses by cells of adaptive immunity offers an advantage over total non-specific cytokine responses. Significantly, the measurement of antigen-specific IFN-γ responses against pathogens or cancer cells, when compared to a treatment group, provides a quantitative measure of how well the treatment works. Measuring antigen-specific IFN-γ responses involves culture of the cells being considered (CD4+ or CD8+ T cells) with antigen presenting cells (APC) and a specific peptide from the target pathogen or cancer cell compared to control cultures without a peptide. After a suitable timeframe, the cytokine released is measured by an ELISPOT assay. The difference in the number of cells secreting IFN-γ, with and without peptide, is a measure of antigen-specific IFN-γ responses. This assay can be applied to other cytokines such as IL-10.
Keywords: IFN-gammaBackground
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons (Gray and Goeddel, 1982). IFN-γ has anti-pathogen, immuno-regulatory, and anti-tumor properties (Schroder et al., 2004) which promotes NK cell activity, increase in antigen presentation, activates inducible nitric oxide synthase, induces the production of IgG2a from activated plasma cells and promotes Th1 differentiation by up-regulating the transcription factor T-bet.
Given the significant role of this cytokine in immune responses, there are several protocols to quantify IFN-γ. Perhaps the simplest measure is an ELISA assay which is used to measure levels of the cytokine in serum samples and tissue culture supernatants by capturing the cytokine with antibodies (Schreiber, 2001). There is also a flow cytometry based-assay where intracellular IFN-γ is detected by flow cytometry following cell-permeabilization (Andersson et al., 1988). The percentage of cells containing the cytokine is usually low, and does not indicate if the protein is functional, if it would be secreted and does not measure if it is in response to a specific target antigen or multiple antigens.
To measure IFN-γ responses to specific antigens, culture assays were developed. Here, CD4+ and CD8+ T cells were stimulated in culture with APC and peptides from the target protein and the supernatants were tested by ELISA for IFN-γ levels (Bradley et al., 1991). Recently, instead of ELISA, several commercial flow cytometry-based bead array assays (e.g., BD Biosciences) are available which offer greater sensitivity to detect low cytokine levels at the nanogram level. However, while the assay can quantify total cytokine secreted, it does not differentiate between a few cells producing a lot of cytokine from a large number of cells secreting little cytokine. The number of cells secreting the cytokine quantifies the number of cells committed to a specific target of immunity. Thus, the enzyme-linked immunospot (ELISPOT) assay is a highly sensitive immunoassay that measures the frequency of cytokine-secreting cells at the single-cell level. An antigen-specific ELISPOT assay allows the quantification of the number of a specific cell type (CD4+ or CD8+ T cells) which secretes IFN-γ in response to a specific antigen (Carvalho et al., 2001; Schmittel et al., 2001)
The IFN-γ–specific antibody on an ELISPOT plate captures the IFN-γ immediately after secretion from the cells with a limit of detection typically around 1 in 100,000 cells. The high sensitivity of the assay makes it particularly useful for studies of the small population of cells found in specific immune responses (Horne-Debets et al., 2013 and 2016; Karunarathne et al., 2016).
Materials and Reagents
- Nitrile gloves
- Sterile 15 ml and 50 ml polypropylene tubes
- Disposable sterile pipettes: 2 ml, 5 ml, 10 ml, 25 ml
- Filters for syringes: 0.45 μM and/or 0.22 μM
- Multiscreen HTS-IP plates (PVDF membrane) (Merck, catalog number: MSIPS4510 )
- Mouse (BioLegend, catalog number: 575402 ) or human (Thermo Fisher Scientific, GibcoTM, catalog number: PHC0026 ) recombinant IL-2
- Microbeads (Miltenyi Biotec)
Note: Catalog numbers depend on the cell type to be tested. - Dynabeads magnetic beads (Thermo Fisher Scientific, USA, https://www.thermofisher.com/kr/en/home/brands/product-brand/dynal.html)
Note: Catalog numbers depend on the cell type tested. - Ethanol
- Peptides or antigen from the protein of choice to stimulate antigen-specific IFN-γ
- Rat anti-mouse IFNγ mAb, clone AN18, purified (capture) (50 μg, Thermo Fisher Scientific, eBioscienceTM, catalog number: 14-7313-81 or 500 μg, Thermo Fisher Scientific, eBioscienceTM, catalog number: 14-7313-85 ) mouse anti-human IFNγ mAb, p clone NIB42, purified (capture) (50 μg, BioLegend, catalog number: 502403 or 500 μg, BioLegend, catalog number: 502404 )
Note: Either titrate or use as recommended by manufacturer. Prepare immediately before coating wells. - Rat anti-mouse IFNγ mAb, clone R4-6A2, biotinylated (detection) (50 μg, Thermo Fisher Scientific, eBioscienceTM, catalog number: 13-7312-81 or 500 μg, Thermo Fisher Scientific, eBioscienceTM, catalog number: 13-7312-85 ) or mouse anti-human IFNγ mAb, clone 4S.B3, biotinylated (detection) (50 μg, BioLegend, catalog number: 502503 or 500 μg, BioLegend, catalog number: 502504 )
Note: Either titrate or use as recommended by manufacturer. - 0.5% BSA
- Streptavidin-horseradish peroxidase (BioLegend, catalog number: 405210 )
- 3-amino-9-ethylcarbazole (AEC) substrate/chromogen (BD, catalog number: 551951 )
- Milli-Q water
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Sodium phosphate dibasic (Na2HPO4)
- Potassium phosphate monobasic (KH2PO4)
- Hydrochloric acid (HCl)
- Sodium bicarbonate (NaHCO3)
- Sodium phosphate (Na2CO3)
- IMDM-1640
- Fetal calf serum (FCS)
Note: Any brand that is suitable for cell culture and is heat inactivated at 56 °C for 30 min. - Penicillin-streptomycin (Tissue culture grade; Life technologies)
- β-mercaptoethanol
- Tween 20 (store at room temperature)
- IMDM culture medium
- L-glutamine (Tissue culture grade; Life technologies)
- Phytohemagglutinin-L (PHA, positive control) stock (Roche Diagnostics, catalog number: 11249738001 ). Refer to Recipes section for details
- Sterile phosphate buffered saline (1x PBS) (see Recipes)
- Coating buffer working solution (see Recipes)
- Blocking solution (see Recipes)
- Anti-IFN-γ capture working solution (see Recipes)
- Biotin-anti-IFN-γ working solution (see Recipes)
- Streptavidin-horseradish peroxidase working solution (see Recipes)
- Tween 20 working solution (see Recipes)
- 10% fetal calf serum in IMDM culture medium (see Recipes)
- PHA stock solution (see Recipes)
Equipment
Note: ELISPOTS on the plate can be manually counted under a dissection microscope, or stereomicroscope (For example, Greenough, high-performance zoom stereomicroscope, SMZ 168-series). Alternatively, there are several specialist automated systems for high throughput screening (AELVIS, Autoimmun Diagnostika, Bio-Sys, Cellular Technology and the Zeiss reader) and there pros and cons discussed elsewhere and beyond the scope of this protocol (Janetzki et al., 2015).
- Waste container
- Gilson pipette and tips: P-2, P-10, P-20, P200, P1000
- Gilson multichannel pipette with matched tips
- Beckman Allegra 12 refrigerated centrifuge (Beckman Coulter, model: Allegra X-12 )
- Class II biohazard hood
- Incubator
- 1 L bottles
Procedure
There are two ways to measure cell type-specific responses. Firstly, isolate total CD4+ and/or CD8+ T cells and culture with dendritic cells (DCs) and the peptide of choice (Karunarathne et al., 2016). T cells are generally enriched from experimental mice using bead based techniques such as Dynabeads magnetic beads (Untouched Mouse T cells kit; Life Technologies, US) or Miltenyi Biotec (CD90.2 T cell isolation; Germany). DCs are immuno-magnetically isolated from naive mice (for use as antigen-presenting cells; APC) using anti-mouse CD11c Microbeads (Miltenyi Biotec; Germany). DCs should not be isolated from experimental mice where DC function is known to be compromised. Both cell types (T cells and DCs) can also be isolated using flow cytometry-based cell sorting. Approximately 2 x 105 T cells, from individual mice, should be co-cultured with 2 x 104 DC, in 4-8 replicate wells per sample with 20 μg/ml peptides, recombinant protein or no peptide, as previously described (Howland et al., 2013). Alternatively, if peptides are specific for CD4+ but not CD8+ T cells and vice versa, then total spleen cell with no additional DCs or total T cells with DCs may be used. These combined cells are then cultured with the specific peptide on ELISPOT plates coated with anti-IFN-γ antibody. The IFN-γ-specific antibody on an ELISPOT plate captures the IFN-γ immediately after secretion from the cells. For human T cells, total PBMCs containing APC and T cells are cultured with the peptide of choice. For the positive control, just add PHA to total cells or isolated T cells without DCs.
- Day 1
- Coat Multiscreen HTS-IP plates with primary antibody diluted in coating buffer:
Before coating the plate with antibody, humidify the membrane in each well with 15 μl of 70% ethanol (in Milli-Q water) for 1 min. Rinse with 150 μl sterile PBS three times before the ethanol evaporates. Then coat plates with 50 μl anti-IFNγ antibody (capture) in sterile coating buffer. Incubate overnight at 4 °C.
Notes: - Plate may be coated 1 week prior to use but must be stored in humid conditions (e.g., box with tissue drenched in sterile water or PBS).
- The number of wells coated with antibody should take into account the all controls listed below and at least 4-8 replicate wells for each sample.
- If possible, it is recommended that a titration of cell numbers (Figure 1), in replicates, be tested to ensure an optimal range of cells for counting.
- Ensure that sodium azide is never added to any buffer, at any step as this stops horseradish peroxidase activity.
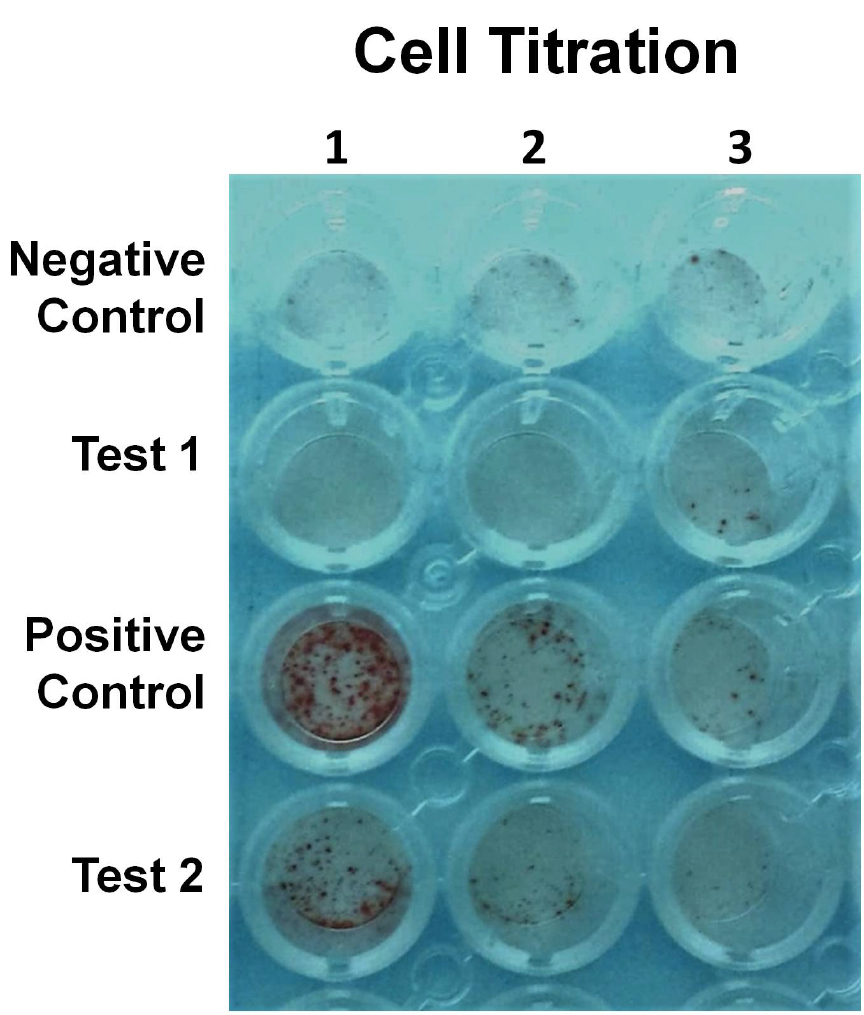
Figure 1. A representative example of an ELISPOT assay. A titration of cell numbers from negative and positive controls and 2 test samples. Test 1 shows no titration with dilution and thus and cell numbers are similar to negative control. Test 2 has declining number of cells with titration indicating IFN-γ-secreting cells. - The following control wells should be incorporated into the assay:
- No antigen stimulation (DC and T cells cultured without peptide).
- Positive control with a lymphocyte mitogen (T cells without DC but with mitogen like PHA at 5-10 μg/ml).
- No cells; no primary antibody.
- No antigen stimulation (DC and T cells cultured without peptide).
- Coat Multiscreen HTS-IP plates with primary antibody diluted in coating buffer:
- Day 2
- Block membrane with blocking solution.
- Remove primary antibody solution.
- Wash off unbound antibody with 150 μl sterile PBS per well, at least twice.
- Block membrane with 150 μl per well of cell culture medium (see Recipes) for at least 2 h at 37 °C.
Note: Keep changing media if it goes deep pink, until orange.
- Remove primary antibody solution.
- Prepare mouse splenocytes (MacPherson et al., 2001; Wykes et al., 2007) or isolate T cells/DCs, suspended in cell culture medium (see Recipes). Plan for each sample to have 4-8 replicate wells.
- For assays using total splenocytes or PBMCs, cells are resuspended in cell culture medium, at final concentrations range of 2 x 106 to 6 x 106 cells/ml for testing at possible testing range of 1 x 105 to 3 x 105 splenocytes or PBMCs per well.
- Isolated T cells are usually resuspended at 4 x 106 cells/ml for testing 2 x 105 T cells cultured with 2 x 104 DC per well.
Note: The desired cell concentration is dependent on the intensity of the immune response. If the expected response is not known, a serial dilution of cell concentrations is recommended. - Antigens are diluted (20 μg/ml) in culture medium eventually containing recombinant IL2 (60 U/ml).
- Cell stimulation
- Discard blocking medium.
- Add 50 μl of the cell suspension and 50 μl of diluted peptide (antigen) per well (final volume: 100 μl/well, corresponding to 10 μg/ml for the antigen and 30 U/ml for recombinant IL2).
Note: To minimize overseeding of the wells, it is recommended to not add more than 3 x 105 cells/well. - Incubate for 18 to 24 h at 37 °C, 5% CO2, 95% humidity.
Note: Do not move the plates while the cells are culturing. This will lead to ‘snail trail’ spots that will not be well defined. Don’t stack the plates if you have more than one to prevent edge effects.
- Block membrane with blocking solution.
- Day 3
- Secondary antibody
- Remove culture medium with cells.
- Wash plate 3 times with PBS and 3 times with PBS/0.01% Tween 20.
Note: Ensure you include Tween 20 in the wash buffer. Some cells will have started attaching after culture overnight. Tween 20 will help wash these off the membrane. Do not use a plate washer at this stage if available. - Dilute biotinylated anti-IFNγ antibody (detection) in PBS/0.5% BSA. Pass the detection antibody mixture through a 0.45 μm or 0.22 μm filter to remove aggregates. Some of the mixture will be lost by filtration and original volume must be scaled up accordingly. Add 50 μl of detection antibody mixture to each well.
Note: Failure to filter secondary antibody may results in non-specific spot formation due to protein aggregates. - Incubate for 2 h at 37 °C, 5% CO2, 95% humidity.
- Then incubate overnight at 4 °C, in a sealed box with tissues moistened with sterile water or PBS which provide humidity.
- Remove culture medium with cells.
- Secondary antibody
- Day 4
- Wash plate 6 times with PBS/0.01% Tween 20.
- Enzyme conjugate and substrate development
- Prepare streptavidin-horseradish peroxidase dilution in PBS.
- Add 50 μl per well of streptavidin-horseradish peroxidase. Incubate for 45 min at room temperature.
Note: Exceeding 1 h incubation with enzyme conjugate could result in increased background color. - Remove streptavidin solution, wash 3 times with PBS/0.01% Tween 20, followed by 3 washes with PBS.
Notes: - The final washes with only PBS are important as Tween 20 will interfere with the spot development.
- The plastic base should be taken off the bottom of the plate to enable thorough washing of the membrane before adding substrate/chromogen. For example, after incubation with the streptavidin horseradish peroxidase conjugate, remove the base and wash both sides of the membrane under running distilled water. This helps to prevent high background as some reagents can leak through the membrane into the bottom tray of the plate.
- Add 75 μl/well AEC substrate (after passing through 0.45 μM or 0.22 μM filter). Incubate for 5 min in the dark (under aluminum foil or in a drawer) and then check for spots. If spots are not clearly visible, incubate again and check regularly. Stop spot development when spots are clearly visible but before background membrane color becomes too dark and the contrast between spot and membrane color is lost.
Note: Optimization of the time of substrate development is critical. Time of development may vary. Over-development will result in increased background. - Stop spot development using running tap water and wash extensively. While washing, remove the bottom of the plate and continue rinsing.
- Press the plate thoroughly to an absorbent tissue paper.
- Let plate dry overnight in the dark. Spot intensity may decrease with exposure to light.
Note: Spots may become sharper if membranes are stored overnight. If storing, wrap membranes in foil.
- Wash plate 6 times with PBS/0.01% Tween 20.
Data analysis
- Spots are counted under a dissection microscope or in an automated ELISPOT reader, and the frequency of secreting cells is calculated:
One cell = One spot
Number of spots = number of cells secreting cytokines
Intensity and size of spot = relative cytokine-secreting ability of cells - Background subtraction
For ELISPOT data analysis, the background value is subtracted from measured results. Each specimen group should have a single background spot mean/median value, calculated from the negative control (T cells and DCs without antigen). Then for each antigen group in the sample, the mean/median for the group is calculated, the background mean/median is then subtracted and the count is normalized by the number of cells per well. - Assay
- Positive should be > 50 spots/106 cells.
- Negative control should ideally be 0 spots/106 cells. If > 0 and < 50 spots/106 cells, subtract the negative control reading from the test count to correct for background staining. If > 50 spots/106 cells, repeat the assay.
- Contamination:
- If one of the replicate wells of one assay condition clearly appears contaminated, ignore that particular well and use the result from the other wells.
- If multiple wells clearly appear to be contaminated, repeat the assay.
- Results
For each test antigen, results are reported as spot forming cells (SFC)/million for human PBMC or SFC/spleen for mouse spleen cells.
Notes
- As horseradish peroxidase activity is completely stopped by sodium azide, please ensure it is never added to any buffer or reagents associated with this assay.
- There are several brands of antibodies available that are suitable for this assay and can even be purchased as antibody pairs. However, each new antibody vial should be tested in a checkerboard titration to determine the optimal dilution (Asai, 2000 #14220).
Recipes
- Sterile phosphate buffered saline (1x PBS)
Start with 800 ml of distilled water, to which add 8 g of NaCl; 0.2 g of KCl; 1.44 g of Na2HPO4 and 0.24 g of KH2PO4
Adjust the pH to 7.4 with HCl
Add distilled water to a total volume of 1 L
Stock is stored in 1 L bottles
Store at room temperature - Coating buffer working solution (0.5 M carbonate-bicarbonate buffer pH 9.6)
3.7 g NaHCO3 and 0.6 g Na2CO3 (anhydrous) made up to 100 ml with distilled water
Make sure that the carbonate bicarbonate is dissolved by gently mixing it until there is no powder residue left and filter sterilized with 0.22 μM filter
Check pH which should be ~9.6 and occasionally needs to be adjusted
Store at room temperature in the dark - Blocking solution
IMDM culture medium
10% fetal calf serum
1% penicillin-streptomycin
0.1% β-mercaptoethanol - Anti-IFN-γ capture working solution
This is the capture antibody. Prepare immediately before coating wells. Need 50 μl per well, at a concentration according to manufacturer's instruction or by titration for each antibody stock - Biotin-anti-IFN-γ working solution
This is the detection antibody. Prepare as in PBS. Need 50 μl per well, at a concentration according to manufacturer's instruction or by titration for each antibody stock - Streptavidin-horseradish peroxidase working solution
Prepare a dilution of the stock in PBS. Need 50 μl per well, at a concentration according to manufacturer's instruction or by titration for each antibody stock - Tween 20 working solution
Add 0.5 ml Tween 20 stock to 1,000 ml 1x PBS
Store at room temperature - 10% fetal calf serum in IMDM culture medium (store at 4 °C in the dark)
- Fetal calf serum stock
Thaw and dispense into 50 ml aliquots. Store at -20 °C - IMDM culture medium (store at 4 °C in the dark)
- L-glutamine stock
Supplied as 50 ml at 200 mM concentration. Store at -20 °C
Thaw and dispense into 1 or 5 ml aliquots depending on volume of culture medium to be prepared later. Store at -20 °C in the dark until needed - Penicillin-streptomycin (5,000 U/ml)
Supplied as 100 ml at 5,000 U/ml concentration. Store at -20 °C
Thaw and dispense into 1 or 5 ml aliquots depending on volume of culture medium to be prepared later. Store at -20 °C in the dark until needed - 10% fetal calf serum in IMDM culture medium (complete medium)
For 100 ml: add 10 ml FCS (a), 1 ml L-glutamine (c), 1 ml penicillin-streptomycin (d), 100 μl β-mercaptoethanol, and to 88 ml IMDM (b). Store at 4 °C in dark - PHA stock solution
Add to 10 ml PBS to the PHA powder for a concentration of 500 μg/ml
Aliquot and freeze in volumes of 10 μl. Store at -80 °C
Notes: - Do not re-freeze thawed PHA.
- PHA (Lectin, positive control) stock is supplied as 5 mg of purified, salt-free lyophilized powder.
- In the assay, use at a final concentration of 5-10 μg/ml. Add 1-2 μl of stock solution to each well containing cells in a 100 μl volume
Acknowledgments
MNW was supported by ARC Future Fellowship and NHMRC. L.R. was supported by Singapore’s A*STAR and NRF Singapore (NRF2007NRF-RF001-226).The authors wish to thank Mr Josh Horne-Debets for the ELISPOT image.
The ELISPOT method was originally developed to detect antigen-specific antibodies from B cells (Czerkinsky et al., 1983) and has over the years been modified by several groups to detect cytokines produced by antigen-specific cells.
References
- Andersson, U., Hallden, G., Persson, U., Hed, J., Moller, G. and DeLey, M. (1988). Enumeration of IFN-γ-producing cells by flow cytometry. Comparison with fluorescence microscopy. J Immunol Methods 112(1): 139-142.
- Bradley, L. M., Duncan, D. D., Tonkonogy, S. and Swain, S. L. (1991). Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J Exp Med 174(3): 547-559.
- Carvalho, L. H., Hafalla, J. C. and Zavala, F. (2001). ELISPOT assay to measure antigen-specific murine CD8+ T cell responses. J Immunol Methods 252(1-2): 207-218.
- Czerkinsky, C. C., Nilsson, L. A., Nygren, H., Ouchterlony, O. and Tarkowski, A. (1983). A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods 65(1-2): 109-121.
- Gray, P. W. and Goeddel, D. V. (1982). Structure of the human immune interferon gene. Nature 298(5877): 859-863.
- Horne-Debets, J. M., Faleiro, R., Karunarathne, D. S., Liu, X. Q., Lineburg, K. E., Poh, C. M., Grotenbreg, G. M., Hill, G. R., MacDonald, K. P., Good, M. F., Renia, L., Ahmed, R., Sharpe, A. H. and Wykes, M. N. (2013). PD-1 dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep 5(5): 1204-1213.
- Horne-Debets, J. M., Karunarathne, D. S., Faleiro, R. J., Poh, C. M., Renia, L. and Wykes, M. N. (2016). Mice lacking Programmed cell death-1 show a role for CD8+ T cells in long-term immunity against blood-stage malaria. Sci Rep 6: 26210.
- Howland, S. W., Poh, C. M., Gun, S. Y., Claser, C., Malleret, B., Shastri, N., Ginhoux, F., Grotenbreg, G. M. and Renia, L. (2013). Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med 5(7): 984-999.
- Janetzki, S., Price, L., Schroeder, H., Britten, C. M., Welters, M. J. and Hoos, A. (2015). Guidelines for the automated evaluation of Elispot assays. Nat Protoc 10(7): 1098-1115.
- Karunarathne, D. S., Horne-Debets, J. M., Huang, J. X., Faleiro, R., Leow, C. Y., Amante, F., Watkins, T. S., Miles, J. J., Dwyer, P. J., Stacey, K. J., Yarski, M., Poh, C. M., Lee, J. S., Cooper, M. A., Renia, L., Richard, D., McCarthy, J. S., Sharpe, A. H. and Wykes, M. N. (2016). Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4+ T cell immunity. Immunity 45(2): 333-345.
- MacPherson, G. G., Wykes, M., Huang, F. P. and Jenkins, C. D. (2001). Isolation of dendritic cells from rat intestinal lymph and spleen. In: Robinson, S. P. and Stagg, A. J. (Eds.). Dendritic Cells Protocols. Humana Press.
- Schmittel, A., Keilholz, U., Bauer, S., Kuhne, U., Stevanovic, S., Thiel, E. and Scheibenbogen, C. (2001). Application of the IFN-γ ELISPOT assay to quantify T cell responses against proteins. J Immunol Methods 247(1-2): 17-24.
- Schreiber, R. D. (2001). Measurement of mouse and human interferon gamma. Curr Protoc Immunol Chapter 6: Unit 6 8.
- Schroder, K., Hertzog, P. J., Ravasi, T. and Hume, D. A. (2004). Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol 75(2): 163-189.
- Wykes, M. N., Liu, X. Q., Jiang, S., Hirunpetcharat, C. and Good, M. F. (2007). Systemic tumor necrosis factor generated during lethal Plasmodium infections impairs dendritic cell function. J Immunol 179(6): 3982-3987.
17823-K
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wykes, M. N. and Rénia, L. (2017). ELISPOT Assay to Measure Peptide-specific IFN-γ Production. Bio-protocol 7(11): e2302. DOI: 10.21769/BioProtoc.2302.
Category
Immunology > Immune cell function > Cytokine
Cell Biology > Cell-based analysis > Protein secretion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











